GET TOP RATED STOCK ALERTS ACTIVE TRADERS DEPEND ON
SIGN UP TODAY FOR FREE NEWS DRIVEN ALERTS

Key step towards limited launch mid-2020
Houston, TX – February 11, 2020 –Soliton, Inc., (NASDAQ: SOLY) (“Soliton” or the “Company”), a medical device company with a novel and proprietary platform technology licensed from The University of Texas on behalf of the MD Anderson Cancer Center (“MD Anderson”), today announced that it has filed for Special 510(k) Premarket Notification with the U.S. Food and Drug Administration (“FDA”) for its Generation II Rapid Acoustic Pulse (“RAP”) device.
The Generation II RAP device delivers the same tattoo-removal therapy as the Generation I device, but is slightly modified for improved ease of use in the physician’s office. The Generation II RAP device constitutes the underlying technology of the RAP device that will be deployed in the limited U.S. commercial launch planned for mid-year 2020. Although, similar technology was utilized in the Company’s pivotal cellulite and proof of concept keloid scar trials, only the tattoo removal indication will be reviewed by the FDA in this submission and enabled during this initial launch.
The Special 510(k) filing states the device is indicated as an accessory to the 1064 nm Q-Switched laser for tattoo removal on the arms, legs and torso in Fitzpatrick Skin Type I-III individuals. Clinical trials have demonstrated that using the Company’s RAP device, in conjunction with a Q-switched laser, allows for multiple passes of laser treatment in a single treatment session, resulting in accelerated fading in comparison to stand-alone laser treatment.
————————
Join our more than 200K fans here to follow the Company: https://soly-investors.com
————————
About Soliton, Inc.
Soliton, Inc. is a medical device company with a novel and proprietary platform technology licensed from MD Anderson. The Company’s first FDA cleared commercial product will use rapid pulses of acoustic shockwaves as an accessory to lasers for the removal of unwanted tattoos. The Company is based in Houston, Texas, and is actively engaged in bringing the Rapid Acoustic Pulse (“RAP”) device to the market. The Company believes this “Soliton” method has the potential to lower tattoo removal costs for patients, while increasing profitability to practitioners, compared to current laser removal methods. Soliton is investigating potential additional capabilities of the RAP technology in clinical and preclinical testing, including the potential to improve the appearance of cellulite by creating mechanical stress at the cellular level and inducing significant collagen growth and the potential to treat keloid and hypertrophic scars by targeting the stiffened environment in the intracellular matrix.
For more information about the Company, please visit: http://www.soliton.com
Forward-Looking Statements
Some of the statements in this release are forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, Section 21E of the Securities Exchange Act of 1934 and the Private Securities Litigation Reform Act of 1995, which involve risks and uncertainties. Forward-looking statements in this press release include, without limitation, the ability to receive clearance of the Special 510(k) and to launch its product in 2020. These statements relate to future events, future expectations, plans and prospects. Although Soliton believes that the expectations reflected in such forward-looking statements are reasonable as of the date made, expectations may prove to have been materially different from the results expressed or implied by such forward-looking statements. Soliton has attempted to identify forward-looking statements by terminology including ”believes,” ”estimates,” ”anticipates,” ”expects,” ”plans,” ”projects,” ”intends,” ”potential,” ”may,” ”could,” ”might,” ”will,” ”should,” ”approximately” or other words that convey uncertainty of future events or outcomes to identify these forward-looking statements. These statements are only predictions and involve known and unknown risks, uncertainties, and other factors, including those discussed under in our SEC filings, including under the heading “Item 1A. Risk Factors” in the Form 10-K for year ended December 31, 2018 we filed with the SEC and updated from time to time in our Form 10-Q filings and in our other public filings with the SEC. Any forward-looking statements contained in this release speak only as of its date. Soliton undertakes no obligation to update any forward-looking statements contained in this release to reflect events or circumstances occurring after its date or to reflect the occurrence of unanticipated events.
Source: Soliton, Inc.

Houston, TX – December 18, 2019 – Soliton, Inc., (NASDAQ: SOLY) (“Soliton” or the “Company”), a medical device company with a novel and proprietary platform technology licensed from The University of Texas on behalf of the MD Anderson Cancer Center (“MD Anderson”), today announced its Rapid Acoustic Pulse (“RAP”) device successfully completed the IEC 60601 safety testing being conducted at SGS, the world’s leading inspection, verification, testing and certification company. The testing was led by a team at Sanmina Corporation, a leading electronics manufacturing services provider.
This safety testing is a requirement of the supplemental 510(k) filing for the Company’s RAP device for tattoo removal, improvement in the appearance of cellulite, keloid (scarring) and additional pipeline indications. The tests are intended to insure that devices meet standard safety metrics to protect users and patients. The supplemental 510(k) filing will provide an update to Soliton’s current FDA 510(k) clearance, which was received in May 2019 for tattoo removal, with respect to the step changes made to the device to improve usability in the field.
Dr. Chris Capelli, Soliton’s President, CEO and co-founder, commented, “We are pleased, but not surprised by the safety testing results of our RAP device. The safety of our second generation device mirrors that of our first, and these results reinforce this. We look forward to submitting this safety data in early 2020, simultaneous with the Special 510k filing of our second generation RAP device. This step is an important one in our plans to commercialize the device being launched for tattoo removal to a select and limited number of dermatologists in mid-2020.”
————————
Join our more than 200K fans here to follow the Company: https://soly-investors.com
————————
About Soliton, Inc.
Soliton, Inc. is a medical device company with a novel and proprietary platform technology licensed from MD Anderson. The Company’s first FDA cleared commercial product will use rapid pulses of acoustic shockwaves as an accessory to lasers for the removal of unwanted tattoos. The Company is based in Houston, Texas, and is actively engaged in bringing the Rapid Acoustic Pulse (“RAP”) device to the market. The Company believes this “Soliton” method has the potential to lower tattoo removal costs for patients, while increasing profitability to practitioners, compared to current laser removal methods. Soliton is investigating potential additional capabilities of the RAP technology in clinical and preclinical testing, including the potential to improve the appearance of cellulite by creating mechanical stress at the cellular level and inducing significant collagen growth and the potential to treat keloid and hypertrophic scars by targeting the stiffened environment in the intracellular matrix.
For more information about the Company, please visit: http://www.soliton.com
Forward-Looking Statements
Some of the statements in this release are forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, Section 21E of the Securities Exchange Act of 1934 and the Private Securities Litigation Reform Act of 1995, which involve risks and uncertainties. Forward-looking statements in this press release include, without limitation, the ability to submit the safety data for the second generation RAP device in early 2020, and to commercialize the device being launched for tattoo removal to a select and limited number of dermatologists in mid-2020. These statements relate to future events, future expectations, plans and prospects. Although Soliton believes that the expectations reflected in such forward-looking statements are reasonable as of the date made, expectations may prove to have been materially different from the results expressed or implied by such forward-looking statements. Soliton has attempted to identify forward-looking statements by terminology including ”believes,” ”estimates,” ”anticipates,” ”expects,” ”plans,” ”projects,” ”intends,” ”potential,” ”may,” ”could,” ”might,” ”will,” ”should,” ”approximately” or other words that convey uncertainty of future events or outcomes to identify these forward-looking statements. These statements are only predictions and involve known and unknown risks, uncertainties, and other factors, including those discussed under in our SEC filings, including under the heading “Item 1A. Risk Factors” in the Form 10-K for year ended December 31, 2018 we filed with the SEC and updated from time to time in our Form 10-Q filings and in our other public filings with the SEC. Any forward-looking statements contained in this release speak only as of its date. Soliton undertakes no obligation to update any forward-looking statements contained in this release to reflect events or circumstances occurring after its date or to reflect the occurrence of unanticipated events.
Source: Soliton, Inc

Results to be Presented at American Society for Dermatologic Surgery Annual Meeting
Houston, TX – October 8, 2019 – Soliton, Inc., (NASDAQ: SOLY) (“Soliton” or the “Company”), a medical device company with a novel and proprietary platform technology licensed from The University of Texas on behalf of the MD Anderson Cancer Center (“MD Anderson”), today announced that the Company’s preliminary proof of concept study results using its Rapid Acoustic Pulse (RAP) Device for the treatment of fibrotic (keloid and hypertrophic) scars has been selected for presentation via abstract at the American Society for Dermatologic Surgery (“ASDS”) Annual Meeting on October 24, 2019. The ASDS Annual Meeting is taking place on October 24-27, 2019 in Chicago, IL.
————————
Join our more than 200K fans here to follow the Company: https://soly-investors.com
————————
“We are pleased to be presenting the results from our initial follow-up visit to attendees at this important dermatology conference,” stated Christopher Capelli, MD, founder, President and CEO of Soliton. “We look forward to being able to share these results with our shareholders and all interested parties as quickly as possible after the conference. We view this proof-of-concept trial as the starting point for demonstrating that our technology could impact other fibrotic disorders. The same mechanism of action at work to reduce keloid and hypertrophic scars could likely extend to such indications as capsular contraction, Peyrone’s Disease, and even liver fibrosis.”
Fibrotic scars, such as keloid and hypertrophic scars, represent wound healing gone awry. Existing published research suggests that factors relating to the wound-healing environment (including tension at the boundary of the scar) can cause fibroblasts to become stuck in a hyper-productive loop, unable to stop the production of collagen that leads to the thickened, raised and dense structures often associated with these fibrotic scars.
The American Osteopathic College of Dermatology estimates that keloids affect around 10 percent of people, whereas hypertrophic scars are more common. Keloid scars are more prevalent among populations with darker pigmentation. Hypertrophic scars affect men and women from any racial group equally, although people between the ages of 10 and 30 years old are more likely to be affected.
Grand View Research estimates the global market for keloid and hypertrophic scars may reach $10.2 billion by 2025. There are few treatment options available for fibrotic scars, which in addition to being disfiguring, can also cause significant discomfort. Currently, the most common treatment is the direct injection of steroids into the scar, however this can require multiple injections and may not be a permanent solution.
About Soliton, Inc.
Soliton, Inc. is a medical device company with a novel and proprietary platform technology licensed from MD Anderson. The Company’s first FDA cleared commercial product will use rapid pulses of acoustic shockwaves as an accessory to lasers for the removal of unwanted tattoos. The Company is based in Houston, Texas, and is actively engaged in bringing the Rapid Acoustic Pulse (“RAP”) device to the market. The Company believes this “Soliton” method has the potential to lower tattoo removal costs for patients, while increasing profitability to practitioners, compared to current laser removal methods. Soliton is investigating potential additional capabilities of the RAP technology in preclinical testing, including the potential to assist existing fat reduction technology in the reduction of fat as well as improving the appearance of cellulite by creating mechanical stress at the cellular level and inducing significant collagen growth.
For more information about the Company, please visit: http://www.soliton.com
Forward-Looking Statements
Some of the statements in this release are forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, Section 21E of the Securities Exchange Act of 1934 and the Private Securities Litigation Reform Act of 1995, which involve risks and uncertainties. Forward-looking statements in this press release include, without limitation, the ability of the Soliton RAP device to demonstrate safety and efficacy in the reduction of keloid and hypertrophic scars, the ability for Soliton to receive FDA clearance for these additional indications and the ability of Soliton to pursue treatment of other fibrotic disorders. These statements relate to future events, future expectations, plans and prospects. Although Soliton believes that the expectations reflected in such forward-looking statements are reasonable as of the date made, expectations may prove to have been materially different from the results expressed or implied by such forward-looking statements. Soliton has attempted to identify forward-looking statements by terminology including ”believes,” ”estimates,” ”anticipates,” ”expects,” ”plans,” ”projects,” ”intends,” ”potential,” ”may,” ”could,” ”might,” ”will,” ”should,” ”approximately” or other words that convey uncertainty of future events or outcomes to identify these forward-looking statements. These statements are only predictions and involve known and unknown risks, uncertainties, and other factors, including those discussed under in our SEC filings, including under the heading “Item 1A. Risk Factors” in the Form 10-K for year ended December 31, 2018 we filed with the SEC and updated from time to time in our Form 10-Q filings and in our other public filings with the SEC. Any forward-looking statements contained in this release speak only as of its date. Soliton undertakes no obligation to update any forward-looking statements contained in this release to reflect events or circumstances occurring after its date or to reflect the occurrence of unanticipated events.
Source: Soliton, Inc.

Successful trials demonstrating Rapid Acoustic Pulse (RAP) technology to reduce keloid and hypertrophic scars may be indicative of a wider range of fibrosis-related indication
Houston, TX – September 10, 2019 – Soliton, Inc., (Nasdaq: SOLY) (“Soliton” or the “Company”), a medical device company with a novel and proprietary platform technology licensed from The University of Texas on behalf of the MD Anderson Cancer Center (“MD Anderson”), today announced that it has selected Clear Dermatology & Aesthetic Center in Scottsdale, AZ as the site for its proof-of-concept (“POC”) clinical trial for the use of its RAP technology for the treatment of keloid and hypertrophic scars. Dr. Brenda LaTowsky will serve as Principal Investigator.
Join our more than 200K fans here to follow the Company: https://soly-investors.com
“We are proud to have Clear Dermatology & Aesthetic Center selected as the clinical site for what could be a ground-breaking new treatment for keloid and hypertrophic scars,” added Dr. Brenda LaTowsky, Clinical Dermatologist and Principal Investigator of the study. “Unfortunately for many of our patients, the currently available treatment options are unacceptable, so a new non-invasive procedure could be quite important.”
Keloid and hypertrophic scars (also called “fibrotic scars”) represent wound healing gone awry. A typical example of a keloid scar would be a post-surgical scar that grows beyond its boundaries. Existing published research suggests that factors relating to the wound-healing environment (including tension at the boundary of the scar) can cause fibroblasts to become stuck in a hyper-productive loop, unable to stop the production of collagen that leads to the thickened, raised and dense structures often associated with these fibrotic scars.
The American Osteopathic College of Dermatology estimates that keloids affect around 10 percent of people, whereas hypertrophic scars are more common. Keloid scars are more prevalent among populations with darker skin pigmentation. Hypertrophic scars affect men and women from any racial group equally, although people between the ages of 10 and 30 years old are more likely to be affected.
Grand View Research estimates the global market for keloid and hypertrophic scars may reach $10.2 billion by 2025. There are few treatment options available for fibrotic scars, which in addition to being disfiguring, can also cause significant discomfort. Currently, the most common treatment is the direct injection of steroids into the scar, however this can require multiple injections and may not be a permanent solution.
“The initial study design calls for a blinded evaluation of treated scars before and 12 weeks after a single RAP treatment session,” stated Dr. Chris Capelli, Soliton CEO and co-founder. “Our hope is to be able to demonstrate a clinically significant reduction in the volume and height of these scars that currently have limited treatment options. Our preclinical studies combined with published literature on the behavior of fibrotic tissue have suggested that our acoustic shockwaves may be capable of disrupting stiff, sclerotic structures created by unwanted fibrosis, of which keloids and hypertrophic scars are just one example. The disruption of stiff structures may help reset the targeted tissue to more normal fibroblast activity for lasting effects.”
The Company’s device for use as an accessory to 1064nm Q-switched lasers for tattoo removal was cleared on May 24, 2019, however technology for the treatment of cellulite and fibrotic scars is investigational and not available for sale in the United States. Soliton will file additional 510(k) applications for the use of RAP technology in these indications.
About Soliton, Inc.
Soliton, Inc. is a medical device company with a novel and proprietary platform technology licensed from MD Anderson. The Company’s first FDA cleared commercial product will use rapid pulses of acoustic shockwaves as an accessory to lasers for the removal of unwanted tattoos. The Company is based in Houston, Texas, and is actively engaged in bringing the Rapid Acoustic Pulse (“RAP”) device to the market. The Company believes this “Soliton” method has the potential to lower tattoo removal costs for patients, while increasing profitability to practitioners, compared to current laser removal methods. Soliton is investigating potential additional capabilities of the RAP technology in preclinical testing, including the potential to improve fibrotic conditions such as keloid or hypertrophic scars as well as improving the appearance of cellulite by creating mechanical stress at the cellular level and inducing significant collagen growth.
For more information about the Company, please visit: http://www.soliton.com
Forward-Looking Statements
Some of the statements in this release are forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, Section 21E of the Securities Exchange Act of 1934 and the Private Securities Litigation Reform Act of 1995, which involve risks and uncertainties. Forward-looking statements in this press release include, without limitation, the ability of the Soliton RAP device to demonstrate safety and efficacy in the reduction keloid and hypertrophic scars, the potential for efficacy in fibrotic scars to extend to other fibrotic disorders, and the ability for Soliton to receive FDA clearance for these additional indications. These statements relate to future events, future expectations, plans and prospects. Although Soliton believes that the expectations reflected in such forward-looking statements are reasonable as of the date made, expectations may prove to have been materially different from the results expressed or implied by such forward-looking statements. Soliton has attempted to identify forward-looking statements by terminology including ”believes,” ”estimates,” ”anticipates,” ”expects,” ”plans,” ”projects,” ”intends,” ”potential,” ”may,” ”could,” ”might,” ”will,” ”should,” ”approximately” or other words that convey uncertainty of future events or outcomes to identify these forward-looking statements. These statements are only predictions and involve known and unknown risks, uncertainties, and other factors, including those discussed under in our SEC filings, including under the heading “Item 1A. Risk Factors” in the Form 10-K for year ended December 31, 2018 we filed with the SEC and updated from time to time in our Form 10-Q filings and in our other public filings with the SEC. Any forward-looking statements contained in this release speak only as of its date. Soliton undertakes no obligation to update any forward-looking statements contained in this release to reflect events or circumstances occurring after its date or to reflect the occurrence of unanticipated events.
SOURCE Soliton, Inc.

Dosing Effect Identified with Acoustic Subcision Treatment of Cellulite
Houston, TX – August 20, 2019 – Soliton, Inc., (NASDAQ: SOLY) (“Soliton” or the “Company”), a medical device company with a novel and proprietary platform technology licensed from The University of Texas on behalf of the MD Anderson Cancer Center (“MD Anderson”), today announced results of preclinical studies which reveal its RAP device appears to deliver increased disruption of the fibrotic septa that contribute to the appearance of cellulite with increased treatment time, implying a dose response to the therapy. The new discovery, referred to as “acoustic subcision,” helps explain the recent proof-of-concept (POC) trial results, which demonstrated a 20-47% improvement in the patient’s Cellulite Severity Score following the use of the Company’s RAP device. The RAP device for the treatment of cellulite is investigational and not available for sale in the United States.
Soliton, Inc. announced results of preclinical studies which reveal its RAP device appears to deliver increased disruption of the fibrotic septa that contribute to the appearance of cellulite with increased treatment time, implying a dose response to the therapy.
Soliton, Inc. announced results of preclinical studies which reveal its RAP device appears to deliver increased disruption of the fibrotic septa that contribute to the appearance of cellulite with increased treatment time, implying a dose response to the therapy.
“We are excited to have demonstrated that an increase in treatment time does indeed appear to result in heightened acoustic subcision,” commented Dr. Chris Capelli, President and CEO of Soliton. “Dose ranging has long been seen in the scientific community as an indication that a technology or drug is responsible for the effect observed. It also increases our optimism regarding the results we will see in our recently started pivotal clinical trial for the treatment of cellulite.”
The term ‘subcision’ normally refers to a surgical procedure used to sever the fibrotic septa using a special hypodermic needle, punctured through the skin, in order to allow the dimpled skin associated with cellulite to return to a flatter, smoother state. This invasive procedure is painful, requiring the use of injected anesthesia and can result in bleeding, bruising and significant post-treatment discomfort and downtime. Our use of the term ‘acoustic subcision’ describes the apparent ability of our RAP technology to do this without ever breaking the skin. What’s more, the procedure should require no anesthesia and importantly, as seen in our clinical trial, there should be no bruising, bleeding or post-treatment discomfort or downtime.
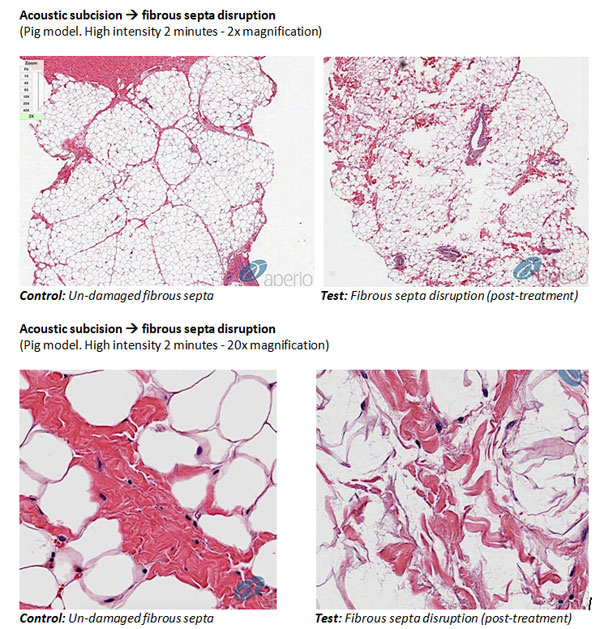
The histology images above show how, in an animal model, untreated skin (left image) contains fibrous structures (called “septa”). Some of these fibrous septa connect the dermis to the muscle layers through the layer of subcutaneous fat. In certain situations, these fibrous septa become stiff (“sclerotic”) and inflexible. As a result, when subcutaneous fat pushes up, the sclerotic fibrous septa hold the skin down causing the appearance of cellulite with deep dimples. Severing the fibrotic septa is currently the only viable means to remove these dimples.
The images moving progressively to the right in the figure above, show tissue samples taken after progressively longer treatments (1 minute, 2 minutes and 3 minutes) with our modified RAP device demonstrating that the mechanical disruption of the septa (shown in red), indeed increases as the time of treatment increases. Importantly, there was little evidence of unintended damage to surrounding tissues such as blood vessels or muscle (not shown). And, in our recent POC clinical trial, 97% of the treatments were rated as having zero pain.
Until now, the only way to have this kind of mechanical disruption of fibrous septa has been through painful invasive procedures. We believe Soliton’s acoustic subcision could change this entirely. The longer-term effect seen in our POC trial is an improvement in the overall appearance of the skin, which we believe is driven through the stimulation of new collagen production.
The Company’s device for use in tattoo removal was cleared on May 24, 2019, however technology for the treatment of cellulite is investigational and not available for sale in the United States. Soliton will file an additional 510(k) application for the use of RAP technology to improve the appearance of cellulite.
About Soliton, Inc.
Soliton, Inc. is a medical device company with a novel and proprietary platform technology licensed from MD Anderson. The Company’s first FDA cleared commercial product will use rapid pulses of acoustic shockwaves as an accessory to lasers for the removal of unwanted tattoos. The Company is based in Houston, Texas, and is actively engaged in bringing the Rapid Acoustic Pulse (“RAP”) device to the market. The Company believes this “Soliton” method has the potential to lower tattoo removal costs for patients, while increasing profitability to practitioners, compared to current laser removal methods. Soliton is investigating potential additional capabilities of the RAP technology in preclinical testing, including the potential to assist existing fat reduction technology in the reduction of fat as well as improving the appearance of cellulite by creating mechanical stress at the cellular level and inducing significant collagen growth.
For more information about the Company, please visit: http://www.soliton.com
Forward-Looking Statements
Some of the statements in this release are forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, Section 21E of the Securities Exchange Act of 1934 and the Private Securities Litigation Reform Act of 1995, which involve risks and uncertainties. Forward-looking statements in this press release include, without limitation, the ability of Soliton’s prototype technology to safely and effectively reduce the appearance of cellulite and its ability to receive regulatory approval for the cellulite indication. These statements relate to future events, future expectations, plans and prospects. Although Soliton believes that the expectations reflected in such forward-looking statements are reasonable as of the date made, expectations may prove to have been materially different from the results expressed or implied by such forward-looking statements. Soliton has attempted to identify forward-looking statements by terminology including ”believes,” ”estimates,” ”anticipates,” ”expects,” ”plans,” ”projects,” ”intends,” ”potential,” ”may,” ”could,” ”might,” ”will,” ”should,” ”approximately” or other words that convey uncertainty of future events or outcomes to identify these forward-looking statements. These statements are only predictions and involve known and unknown risks, uncertainties, and other factors, including those discussed under in our SEC filings, including under the heading “Item 1A. Risk Factors” in the Form 10-K for year ended December 31, 2018 we filed with the SEC and updated from time to time in our Form 10-Q filings and in our other public filings with the SEC. Any forward-looking statements contained in this release speak only as of its date. Soliton undertakes no obligation to update any forward-looking statements contained in this release to reflect events or circumstances occurring after its date or to reflect the occurrence of unanticipated events.
Source: Soliton, Inc.

Houston, TX – August 14, 2019 –- Soliton, Inc., (NASDAQ: SOLY) (“Soliton” or the “Company”), a medical device company with a novel and proprietary platform technology licensed from The University of Texas on behalf of the MD Anderson Cancer Center (“MD Anderson”), today announced that the company has treated the first patients in its pivotal cellulite trial.
————————
Join our more than 200K fans here to follow the Company: https://soly-investors.com
————————
Dr. Chris Capelli, President, CEO and co-founder of Soliton, commented, “Patient treatments have now begun at the Chicago site.” He continued, “It is incredibly exciting to have arrived at this point of the study. A tremendous amount of work goes into preparing for a study of this magnitude and treating the first patients is quite rewarding.”
Cellulite affects up to 90% of women and over a billion dollars per year is spent on treatment in the U.S. Results from our initial proof of concept clinical trial suggest the potential for a new approach to treating cellulite. In the proof of concept trial, the Soliton Rapid Acoustic Pulse (“RAP”) device was applied to the surface of the patients’ skin for a single 20-minute, non-invasive treatment. The treatments required no anesthesia, caused no bruising, swelling or infection, and were evaluated by the trial participants as a “0” on a pain scale of 0-10 in 97% of the treatments. None of the patients experienced any post-treatment downtime. The Soliton device used in this trial has not been reviewed or cleared by the FDA for marketing and, accordingly, none of the information in this press release is intended to promote the sale or use of the device. The device is investigational and is not available for sale in the United States.
About Soliton, Inc.
Soliton, Inc. is a medical device company with a novel and proprietary platform technology licensed from MD Anderson. The Company’s first FDA cleared commercial product will use rapid pulses of acoustic shockwaves as an accessory to lasers for the removal of unwanted tattoos. The Company is based in Houston, Texas, and is actively engaged in bringing the Rapid Acoustic Pulse (“RAP”) device to the market. The Company believes this “Soliton” method has the potential to lower tattoo removal costs for patients, while increasing profitability to practitioners, compared to current laser removal methods. Soliton is investigating potential additional capabilities of the RAP technology in preclinical testing, including the potential to assist existing fat reduction technology in the reduction of fat as well as improving the appearance of cellulite by creating mechanical stress at the cellular level and inducing significant collagen growth.
For more information about the Company, please visit: http://www.soliton.com
Forward-Looking Statements
Some of the statements in this release are forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, Section 21E of the Securities Exchange Act of 1934 and the Private Securities Litigation Reform Act of 1995, which involve risks and uncertainties. Forward-looking statements in this press release include, without limitation, the ability of Soliton’s acoustic shockwave device to reduce cellulite in the larger clinical trial or to receive FDA clearance for the cellulite indication. These statements relate to future events, future expectations, plans and prospects. Although Soliton believes that the expectations reflected in such forward-looking statements are reasonable as of the date made, expectations may prove to have been materially different from the results expressed or implied by such forward-looking statements. Soliton has attempted to identify forward-looking statements by terminology including ”believes,” ”estimates,” ”anticipates,” ”expects,” ”plans,” ”projects,” ”intends,” ”potential,” ”may,” ”could,” ”might,” ”will,” ”should,” ”approximately” or other words that convey uncertainty of future events or outcomes to identify these forward-looking statements. These statements are only predictions and involve known and unknown risks, uncertainties, and other factors, including those discussed under in our SEC filings, including under the heading “Item 1A. Risk Factors” in the Form 10-K for year ended December 31, 2018 we filed with the SEC and updated from time to time in our Form 10-Q filings and in our other public filings with the SEC. Any forward-looking statements contained in this release speak only as of its date. Soliton undertakes no obligation to update any forward-looking statements contained in this release to reflect events or circumstances occurring after its date or to reflect the occurrence of unanticipated events.
Source: Soliton, Inc

Houston, TX – August 12, 2019 – Soliton, Inc., (NASDAQ: SOLY) (“Soliton” or the “Company”), a medical device company with a novel and proprietary platform technology licensed from The University of Texas on behalf of the MD Anderson Cancer Center (“MD Anderson”), today announced that the company has initiated patient recruitment for its upcoming pivotal cellulite trial across all four trial sites. The Company is seeking approximately 60 patients to enroll in the trial. Each trial site is responsible for reviewing pospective patients to determine if enrollment criteria is met by the individual.
————————
Join our more than 200K fans here to follow the Company: https://soly-investors.com
————————
Dr. Chris Capelli, President, CEO and co-founder of Soliton, commented, “Patient recruitment is now active in Boston, Washington, D.C., Chicago and Scottsdale.” He continued, “We anticipate enrolling our targeted patient population quickly and moving rapidly towards patient treatment in these four trial sites. We look forward to the tabulation of the treatment results and the potential for a dramatic advance in the treatment of cellulite.”
Cellulite affects up to 90% of women and over a billion dollars per year is spent on treatment in the U.S. Results from our initial proof of concept clinical trial suggest the potential for a new approach to treating cellulite. In the proof of concept trial, the Soliton Rapid Acoustic Pulse (“RAP”) device was applied to the surface of the patients’ skin for a single 20-minute, non-invasive treatment. The treatments required no anesthesia, caused no bruising, swelling or infection, and were evaluated by the trial participants as a “0” on a pain scale of 0-10 in 97% of the treatments. None of the patients experienced any post-treatment downtime. The Soliton device used in this trial has not been reviewed or cleared by the FDA for marketing and, accordingly, none of the information in this press release is intended to promote the sale or use of the device. The device is investigational and is not available for sale in the United States.
About Soliton, Inc.
Soliton, Inc. is a medical device company with a novel and proprietary platform technology licensed from MD Anderson. The Company’s first FDA cleared commercial product will use rapid pulses of acoustic shockwaves as an accessory to lasers for the removal of unwanted tattoos. The Company is based in Houston, Texas, and is actively engaged in bringing the Rapid Acoustic Pulse (“RAP”) device to the market. The Company believes this “Soliton” method has the potential to lower tattoo removal costs for patients, while increasing profitability to practitioners, compared to current laser removal methods. Soliton is investigating potential additional capabilities of the RAP technology in preclinical testing, including the potential to assist existing fat reduction technology in the reduction of fat as well as improving the appearance of cellulite by creating mechanical stress at the cellular level and inducing significant collagen growth.
For more information about the Company, please visit: http://www.soliton.com
Forward-Looking Statements
Some of the statements in this release are forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, Section 21E of the Securities Exchange Act of 1934 and the Private Securities Litigation Reform Act of 1995, which involve risks and uncertainties. Forward-looking statements in this press release include, without limitation, the ability of Soliton to successfully recruit patients for the pivotal cellulite trial or to receive FDA clearance for the cellulite indication and the ability of Soliton to commence treatments in the pivotal clinical trial within the next few months. These statements relate to future events, future expectations, plans and prospects. Although Soliton believes that the expectations reflected in such forward-looking statements are reasonable as of the date made, expectations may prove to have been materially different from the results expressed or implied by such forward-looking statements. Soliton has attempted to identify forward-looking statements by terminology including ”believes,” ”estimates,” ”anticipates,” ”expects,” ”plans,” ”projects,” ”intends,” ”potential,” ”may,” ”could,” ”might,” ”will,” ”should,” ”approximately” or other words that convey uncertainty of future events or outcomes to identify these forward-looking statements. These statements are only predictions and involve known and unknown risks, uncertainties, and other factors, including those discussed under in our SEC filings, including under the heading “Item 1A. Risk Factors” in the Form 10-K for year ended December 31, 2018 we filed with the SEC and updated from time to time in our Form 10-Q filings and in our other public filings with the SEC. Any forward-looking statements contained in this release speak only as of its date. Soliton undertakes no obligation to update any forward-looking statements contained in this release to reflect events or circumstances occurring after its date or to reflect the occurrence of unanticipated events.
Source: Soliton, Inc.
RAP technology combines selective disruption of fibrotic structures with production of new collagen in single non-invasive procedure
Houston, TX – June 4, 2019 – Soliton, Inc., (NASDAQ:SOLY) (“Soliton” or the “Company”), a medical device company with a novel and proprietary platform technology licensed from The University of Texas on behalf of the MD Anderson Cancer Center (“MD Anderson”), today announced results of preclinical studies of the use of a new version of its acoustic pulse technology which reveal its RAP device appears to be capable of selective disruption of the fibrotic septa that contribute to the appearance of cellulite. The new discovery, referred to as “acoustic subcision,” helps explain the recent proof-of-concept trial results showing an improvement in the appearance of cellulite following use of the Company’s RAP device. Together with the device’s demonstrated ability to stimulate new collagen production in animal models, this represents what the Company believes is a potentially important new way to treat cellulite and improve the appearance of the skin. The RAP device for the treatment of cellulite is investigational and not available for sale in the United States.
“While the proof-of-concept clinical trial results we have recently announced regarding the improvement in the appearance of cellulite have been very encouraging,” commented Dr. Chris Capelli, President and CEO of Soliton, “Understanding the basic science and biology behind these results is very important. We believe the discovery we are outlining here helps explain the promising results we saw from this trial, as well as setting the foundation for a platform technology that may have potential in a number of aesthetic indications.”
The term ‘subcision’ normally refers to a surgical procedure used to sever the fibrotic septa using a special hypodermic needle, punctured through the skin, in order to allow the dimpled skin associated with cellulite to return to a flatter, smoother state. This procedure is typically painful enough that anesthesia is required. Futhermore, this surgical procedure can result in extensive bruising, bleeding or post-treatment discomfort and downtime. Our use of the term ‘acoustic subcision’ describes the apparent ability of our RAP technology to do this without ever breaking the skin. What’s more, the procedure should require no anesthesia and importantly, as seen in our clinical trial, there should be no bruising, bleeding or post-treatment discomfort or downtime.
The histologically images below show how, in an animal model, untreated skin (left image) contains thin, flexible, fibrous structures (called “septa”). Some of these fibrous septa connect the dermis to the muscle layers through the layer of subcutaneous fat. In certain situations, these fibrous septa become stiff (“sclerotic”) and inflexible. As a result, when subcutaneous fat pushes up, the sclerotic fibrous septa hold the skin down causing the appearance of cellulite with deep dimples. Severing the fibrotic septa is currently the only viable means to remove these dimples.
The images on the right in the figure below show a tissue sample taken after a single 2-minute treatment with our modified RAP device demonstrating selective mechanical disruption of the septa. Importantly, there was little evidence of unintended damage to surrounding tissues such as blood vessels or muscle (not shown). And, in our recent proof of concept clinical trial, 97% of the treatments were rated as having zero pain.

Until now, the only way to have this kind of mechanical disruption of fibrous septa has been through painful invasive procedures. We believe Soliton’s acoustic subcision could change all that. The longer-term effect seen in our proof of concept trial is an improvement in the overall appearance of the skin we believe is driven through the stimulation of collagen production.
Collagen is a key component in the extracellular matrix (“ECM”) that help keeps skin strong and smooth. We believe our higher-powered prototype RAP device promotes collagen production through the body’s repair of the mechanically disrupted septa. Preclinical testing indicates that new collagen is being produced which results in the formation of new and thicker septa throughout the subcutaneous fat layer. The Company believes the impact of this response in humans could further provide improvements in the appearance of cellulite.
As shown in the histolotolgy images below, the septa throughout the subcutaneous fat layer demonstrate increased thickening over time as a result of the new acoustic treatments. The histology image on the left was prior to any treatment. The image in the middle was after a single acoustic treatment with the prototype device and the histology image on the far right was after multiple treatments with the device. Soliton believes the development of new and thicker septa could lead to increased integrity and uniformity of the skin.
Skin strengthening in Soliton animal model
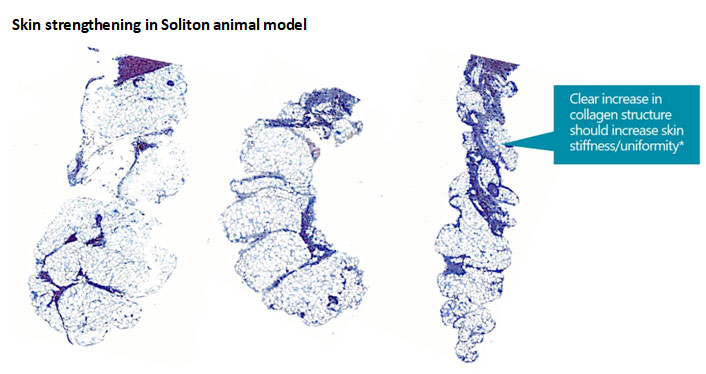
“We believe the ability to promote production of new collagen is a major finding and critical to the ability of this new technology to potentially improve the appearance of cellulite”, commented Dr. Chris Capelli, Soliton’s President, CEO and co-founder.
Dr. Capelli concluded: “An important message here is to understand the potential value of the combined effects – the nearly immediate effect of acoustic subcision to mechanically disrupt sclerotic septa leading to the improvement in the appearance of cellulite dimples and ridges and the longer-term effect of increased collagen production which has the potential to improve the overall smoothness of the skin. More clinical testing is planned to validate these early results”.
The Company’s device for use in tattoo removal was cleared on May 24, 2019, however technology for the treatment of cellulite is investigational and not available for sale in the United States. Soliton will file an additional 510(k) application for the use of RAP technology to improve the appearance of cellulite.
About Soliton, Inc.
Soliton, Inc. is a medical device company with a novel and proprietary platform technology licensed from MD Anderson. The Company’s first FDA cleared commercial product will use rapid pulses of acoustic shockwaves as an accessory to lasers for the removal of unwanted tattoos. The Company is based in Houston, Texas, and is actively engaged in bringing the Rapid Acoustic Pulse (“RAP”) device to the market. The Company believes this “Soliton” method has the potential to lower tattoo removal costs for patients, while increasing profitability to practitioners, compared to current laser removal methods. Soliton is investigating potential additional capabilities of the RAP technology in preclinical testing, including the potential to assist existing fat reduction technology in the reduction of fat as well as improving the appearance of cellulite by creating mechanical stress at the cellular level and inducing significant collagen growth.
For more information about the Company, please visit: http://www.soliton.com
Forward-Looking Statements
Some of the statements in this release are forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, Section 21E of the Securities Exchange Act of 1934 and the Private Securities Litigation Reform Act of 1995, which involve risks and uncertainties. Forward-looking statements in this press release include, without limitation, the ability of Soliton’s prototype technology to safely and effectively reduce the appearance of cellulite and its ability to receive regulatory approval for the cellulite indication. These statements relate to future events, future expectations, plans and prospects. Although Soliton believes that the expectations reflected in such forward-looking statements are reasonable as of the date made, expectations may prove to have been materially different from the results expressed or implied by such forward-looking statements. Soliton has attempted to identify forward-looking statements by terminology including ”believes,” ”estimates,” ”anticipates,” ”expects,” ”plans,” ”projects,” ”intends,” ”potential,” ”may,” ”could,” ”might,” ”will,” ”should,” ”approximately” or other words that convey uncertainty of future events or outcomes to identify these forward-looking statements. These statements are only predictions and involve known and unknown risks, uncertainties, and other factors, including those discussed under in our SEC filings, including under the heading “Item 1A. Risk Factors” in the Form 10-K for year ended December 31, 2018 we filed with the SEC and updated from time to time in our Form 10-Q filings and in our other public filings with the SEC. Any forward-looking statements contained in this release speak only as of its date. Soliton undertakes no obligation to update any forward-looking statements contained in this release to reflect events or circumstances occurring after its date or to reflect the occurrence of unanticipated events.
CONTACT:
Joe Dorame, Joe Diaz & Robert Blum
Lytham Partners, LLC
602-889-9700
Source: Soliton, Inc.

Proof of Concept Trial Reveal Significant Reduction in Cellulite with No Bruising, No Swelling and No Downtime after single 20-Minute non-invasive Procedure; Justifies proceeding with Pivotal Trial.
Houston, TX – May 29, 2019 – Soliton, Inc., (Nasdaq: SOLY) (“Soliton” or the “Company”), a medical device company with a novel and proprietary platform technology licensed from The University of Texas on behalf of the MD Anderson Cancer Center (“MD Anderson”), today announced its decision to move forward with a pivotal trial in cellulite after positive data from its proof of concept clinical trial for the reduction of cellulite (the “POC Trial”) confirmed.
The Pivotal cellulite study is being designed to take place at multiple clinical sites across the country with between 45 and 60 patients to be treated in the study. We hope to begin the study within the next three months.
Cellulite affects up to 90% of women and over a billion dollars per year is spent on treatment in the U.S. Now, results from this Trial suggest the potential for a totally new approach to treating cellulite. In a single 20-minute, non-invasive treatment, the Rapid Acoustic Pulse (RAP) device was applied to the surface of the patients’ skin. The treatments required no anesthesia, caused no bruising, swelling or bleeding, and were evaluated as relatively painless by the trial participants, none of whom experienced any post-treatment discomfort or downtime. The data was originally presented at the SCALE (Symposium for Cosmetic Advances and Laser Education) conference in Nashville, Tennessee, by Dr. Elizabeth Tanzi on May 11, 2019.
The POC Trial involved a study of five patients with moderate to severe cellulite, each treated on their thighs, with a new higher-powered version of Soliton’s RAP device. While the Company’s RAP device intended to assist in tattoo removal was recently cleared by the FDA, this higher-powered version will require a new application for clearance for the cellulite indication. In the POC Trial, approximately 97% of treatments were rated 0 on a 0 to 10-point pain scale (with 0 being no pain). At the end of the 12-week POC Trial, in a blinded review by doctors of before and after photos, 100 percent correctly identified which photo was the “after.” The three blinded reviewers, who are trained in the use of the cellulite severity scoring system, scored the before and after photos using the 5-point system. 100% of the patients showed clinical improvement. The range of improvement in cellulite severity score was 20% to 47% and the average improvement for all patients was nearly a 30% improvement (1.24 reduction on the 0 to 5-point cellulite severity scale). As a point of reference, the only FDA approved method for long-term reduction of cellulite is an invasive treatment called Cellfina that produced an average improvement on the same scale of about 2 points. However, this is a procedure requiring topical anesthesia, penetration of the skin and involves potential bleeding, bruising and significant post-treatment discomfort and downtime. We believe Soliton RAP involves none of these potential negatives.
“The market for non-invasive cellulite treatments is about $1 billion in the U.S., so it is clear that many women who are affected by the condition are interested in finding ways to reduce or eliminate it,” said Walter Klemp, co-founder and Executive Chairman of Soliton. “The very encouraging results of the POC Trial are driving our decision to launch the pivotal trial as quickly as possible and suggest we may be able to significantly improve the appearance of cellulite with a single completely non-invasive procedure. The procedure requires no recovery time and avoids the risks that go with even minimally invasive surgery.”
How the Soliton Device Treats Cellulite
Although cellulite has many contributing factors, a primary cause of the deep dimples and ridges associated with cellulite is the presence of sclerotic septa connecting the dermis to the body’s fascia through the layer of subcutaneous fat. Septa normally provide structure and uniformity to the skin, but over time they can lose uniformity and become stiff and less resilient with larger pockets of subcutaneous fat in-between. As the presence of subcutaneous fat increases, it can push up against the dermis causing it to bulge between these septa, leaving dimples and ridges where the septa refuse to yield.
Superficial therapies are largely ineffective at changing this condition and severing the offending septa is the most effective therapy. Currently, the only method cleared by the FDA for long-term reduction of cellulite is called Cellfina and involves the insertion of a blade or lance into the skin below the dermis. The blade is then moved from side to side in a sweeping motion to cut any septa in its path.
While this “invasive subcision” is immediately effective at removing or reducing deep dimples and ridges, it is also quite painful, requiring the subcutaneous injection of anesthesia. It can also cause bleeding and bruising and significant patient downtime due to ongoing soreness as the wounds from these skin penetrations and cutting actions heal. In addition, this method does very little to smooth or tighten the skin to remove the “orange peel” or “cottage cheese” appearance often associated with cellulite.
Soliton has created a new form of acoustic pulses, which was recently awarded “Best in Show” by the American Society for Laser Medicine and Surgery (ASLMS), that it has shown in animal models are capable of selective disruption of sclerotic septa without ever penetrating the skin. The procedure creates no bleeding or bruising and results in no post-treatment discomfort or downtime.
We believe the disrupted septa resulting from the acoustic pulses induces increased collagen production that thickens and tightens the skin over time. More normal and robust septa are rebuilt with the skin in a smoother position.
“We believe this could represent a new approach to reducing cellulite, which could represent a significant change from the currently available invasive and non-invasive treatments, many of which only work with surface dimpling and can’t help the big dimples in the skin that many want to eliminate,” explained Dr. Chris Capelli, President, CEO, and co-founder of Soliton. “This new approach uses very fast, compressed acoustic pulses in the form of shockwaves and our data suggests that the acoustic pulses may be capable of achieving results previously only thought possible with invasive therapies.”
The Trial was conducted by Dr. Michael Kaminer at SkinCare Physicians in Boston in collaboration with Dr. Elizabeth Tanzi of Capital Laser and Skin Care.
Dr. Kaminer stated: “Having led the clinical development of Cellfina, the only treatment with FDA clearance for producing long-term reduction of cellulite, I am particularly aware of the unmet need in the treatment of cellulite. Having a non-invasive alternative treatment could provide a real benefit for patients while also expanding the breadth of services offered by clinicians.”
Drs. Kaminer and Tanzi are members of Soliton’s Scientific Advisory Board.
About Soliton, Inc.
Soliton, Inc. is a medical device company with a novel and proprietary platform technology licensed from MD Anderson. The Company’s first planned commercial product is designed to use rapid pulses of designed acoustic shockwaves in conjunction with existing lasers to accelerate the removal of unwanted tattoos (RAP device). In addition, higher energy versions of acoustic pulse devices are in early stages of development for potential stand-alone treatment of cellulite and other indications. Both products are investigational and are not available for sale in the United States.
For more information about the Company, please visit: http://www.soliton.com
Forward-Looking Statements
Some of the statements in this release are forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, Section 21E of the Securities Exchange Act of 1934 and the Private Securities Litigation Reform Act of 1995, which involve risks and uncertainties. Forward-looking statements in this press release include, without limitation, the ability of Soliton’s acoustic shockwave device to prove safe and effective for reducing cellulite in larger clinical trials and the timing of the commencement of the pivotal clinical trial. These statements relate to future events, future expectations, plans and prospects. Although Soliton believes that the expectations reflected in such forward-looking statements are reasonable as of the date made, expectations may prove to have been materially different from the results expressed or implied by such forward-looking statements. Soliton has attempted to identify forward-looking statements by terminology including ”believes,” ”estimates,” ”anticipates,” ”expects,” ”plans,” ”projects,” ”intends,” ”potential,” ”may,” ”could,” ”might,” ”will,” ”should,” ”approximately” or other words that convey uncertainty of future events or outcomes to identify these forward-looking statements. These statements are only predictions and involve known and unknown risks, uncertainties, and other factors, including those discussed under in our SEC filings, including under the heading “Item 1A. Risk Factors” in the Form 10-K for year ended December 31, 2018 we filed with the SEC and updated from time to time in our Form 10-Q filings and in our other public filings with the SEC. Any forward-looking statements contained in this release speak only as of its date. Soliton undertakes no obligation to update any forward-looking statements contained in this release to reflect events or circumstances occurring after its date or to reflect the occurrence of unanticipated events.
CONTACT: Joe Dorame, Joe Diaz & Robert Blum
Lytham Partners, LLC
602-889-9700
Source: Soliton, Inc.

Houston, TX – May 28, 2019 – Soliton, Inc., (Nasdaq: SOLY) (“Soliton” or the “Company”), a medical device company with a novel and proprietary platform technology licensed from The University of Texas on behalf of the MD Anderson Cancer Center (“MD Anderson”), today announced that it has received clearance from the U.S. Food & Drug Administration (“FDA”) to market its Rapid Acoustic Pulse (“RAP”) device for tattoo removal. The device is indicated as an accessory to the 1064 nm Q-Switched laser for black ink tattoo removal on the arms, legs and torso in Fitzpatrick Skin Type I-III individuals.
Join our more than 200K fans here to follow the Company: https://soly-investors.com
“Receiving clearance from the FDA, while inline with our expectations, is nonetheless gratifying and validating, representing a bedrock for the commercialization plan of our RAP technology,” commented Dr. Chris Capelli, President, CEO and co-founder of Soliton.
“This clearance to market allows us to begin the transition from R&D to expanded product development and commercialization within the tattoo removal segment.
Development of our breakthrough shockwave device, which was recently awarded “Best in Show” by the American Society for Laser Medicine and Surgery (ASLMS), has taken us over 5 years and more than $25 million in research and development to create. This required collaborating with a wide range of industry-leading experts, including engineers responsible for the Space Shuttle ignition system, scientists specializing in the plasma physics involved in nuclear fusion and physicysts specializing in acoustic engineering.
Our device, which is now covered by 8 patent families with 68 patents issued or pending, safely converts 3,000 volts at 3,000 amps (9,000,000 watts of power) into finely-controlled acoustic shockwaves at a rate of up to 100 pulses per second through our replaceable treatment cartridge. While this is a significant amount of power being converted into an acoustic pulse, it is extremely brief in time, with both a very short rise time and a similarly short fall time. This results in microscopic mechanical disruption to the targeted cellular level structures and vacuoles. Importantly, the negative pressure component of each acoustic pulse is attenuated so therapy can be provided without creating the cavitation, heat or collateral tissue damage that would give the patient the sensation of significant pain. As a result, Soliton’s RAP technolocy can provide meaningful results with little potential for bruising or other treatment related downtime.
In the clinical trials submitted to the FDA as part of the 510(k) application that was just cleared, we demonstrated that our RAP device can enable tattoo removal in just 2-3 office visits. In contrast, a separate independent study of 397 tattoo owners, demonstrated that the standard of care laser-only method required 10 or more office visits to achieve acceptable results.
Taking into consideration that the tattoo removal industry is estimated to grow to approximately $4.8 billion annually by 2023, we are eager to further our commercialization plans and move towards our launch and revenue generation as early the first half of 2020.
Importantly, this FDA clearance represents only the first of many indications we are pursuing with our RAP technology. We believe our RAP technology offers the potential for significant breakthrough treatments in a variety of aesthetic market segments. We look forward to building upon this initial FDA clearance in combination with compelling results from various clinical trials in these other aesthetic segments to advance the influence of our RAP technology.”
About Soliton, Inc.
Soliton, Inc. is a medical device company with a novel and proprietary platform technology licensed from MD Anderson. The Company’s first planned commercial product is designed to use rapid pulses of designed acoustic shockwaves in conjunction with existing lasers to accelerate the removal of unwanted tattoos (RAP device). In addition, higher energy versions of acoustic pulse devices are in early stages of development for potential stand-alone treatment of cellulite and other indications. Both products are investigational and are not available for sale in the United States.
For more information about the Company, please visit: http://www.soliton.com
Forward-Looking Statements
Some of the statements in this release are forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, Section 21E of the Securities Exchange Act of 1934 and the Private Securities Litigation Reform Act of 1995, which involve risks and uncertainties. Forward-looking statements in this press release include, without limitation, the ability of the Soliton RAP technology to prove safe and effective for our targeted indications and to achieve FDA clearance for this technology. These statements relate to future events, future expectations, plans and prospects. Although Soliton believes that the expectations reflected in such forward-looking statements are reasonable as of the date made, expectations may prove to have been materially different from the results expressed or implied by such forward-looking statements. Soliton has attempted to identify forward-looking statements by terminology including ”believes,” ”estimates,” ”anticipates,” ”expects,” ”plans,” ”projects,” ”intends,” ”potential,” ”may,” ”could,” ”might,” ”will,” ”should,” ”approximately” or other words that convey uncertainty of future events or outcomes to identify these forward-looking statements. These statements are only predictions and involve known and unknown risks, uncertainties, and other factors, including those discussed under in our SEC filings, including under the heading “Item 1A. Risk Factors” in the Form 10-K for year ended December 31, 2018 we filed with the SEC and updated from time to time in our Form 10-Q filings and in our other public filings with the SEC. Any forward-looking statements contained in this release speak only as of its date. Soliton undertakes no obligation to update any forward-looking statements contained in this release to reflect events or circumstances occurring after its date or to reflect the occurrence of unanticipated events.
SOURCE Soliton, Inc.

Houston, TX – May 16, 2019 –Soliton, Inc., (NASDAQ: SOLY) (“Soliton” or the “Company”), a medical device company with a novel and proprietary platform technology licensed from The University of Texas on behalf of the MD Anderson Cancer Center (“MD Anderson”), today discussed the use of its Rapid Accoustic Pulse Technology for the removal of tattoos using the capability of the device to clear the vacuoles that result from the use of a laser to ablate tattoo ink.
————————
Join our more than 200K fans here to follow the Company: https://soly-investors.com
————————
A problem arises the moment that laser light contacts ink particles within its path. Almost instantly, a plasma event occurs that quickly results in the formation of steam vacuoles in the skin. These vacuoles appear white in color and result in “optical scattering” that immediately blocks any additional laser energy from reaching ink particles below the vacuoles. Biopsies taken shortly after laser treatment reveal that, in addition to surface vacuoles that clear within an hour or less, deeper “dermal vacuoles” have also formed and shield the remaining particles from subsequent laser passes. Our studies have shown that these deep dermal vacuoles persist for up to 48 hours. Until those vacuoles are gone, we believe subsequent laser passes will have very little effect.
“When you apply the RAP device immediately following a laser treatment, histology reveals that both surface vacuoles and the deep dermal vacuoles are dispersed, allowing lasers to again have line of sight access to pigment particles,” said Dr. Christopher Capelli, President, CEO and Co-Founder of Soliton, Inc. “Thus, using the Soliton RAP device immediately after a laser treatment allows for a subsequent laser treatment in the same office visit, and this cycle may be repeated multiple times.” The RAP device has not been cleared by the FDA.
“On average, a typical black tattoo can require 10 office visits to achieve acceptable removal. Our studies have shown this can now be reduced to just 2-3 visits, and we believe this will have a significant impact on the tattoo removal market, which is predicted to reach $4.3 billion by 2023,” said Walter Klemp, Executive Chairman of Soliton, Inc.
About Soliton, Inc.
Soliton, Inc. is a medical device company with a novel and proprietary platform technology licensed from MD Anderson. The Company’s first planned commercial product is designed to use rapid pulses of designed acoustic shockwaves in conjunction with existing lasers to reduce the number of treatment visits to remove unwanted tattoos (RAP device). In addition, higher energy versions of acoustic pulse devices are in early stages of development for potential stand-alone treatment of cellulite and other indications. Both products are investigational and are not available for sale in the United States.
For more information about the Company, please visit: http://www.soliton.com
Forward-Looking Statements
Some of the statements in this release are forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, Section 21E of the Securities Exchange Act of 1934 and the Private Securities Litigation Reform Act of 1995, which involve risks and uncertainties. Forward-looking statements in this press release include, without limitation, the ability of the Soliton RAP technology, to prove safe and effective at reducing office visits required to remove a tattoo, for the Company’s devices to achieve FDA clearance for the tattoo indications and the projected size of the tattoo removal market. These statements relate to future events, future expectations, plans and prospects. Although Soliton believes that the expectations reflected in such forward-looking statements are reasonable as of the date made, expectations may prove to have been materially different from the results expressed or implied by such forward-looking statements. Soliton has attempted to identify forward-looking statements by terminology including ”believes,” ”estimates,” ”anticipates,” ”expects,” ”plans,” ”projects,” ”intends,” ”potential,” ”may,” ”could,” ”might,” ”will,” ”should,” ”approximately” or other words that convey uncertainty of future events or outcomes to identify these forward-looking statements. These statements are only predictions and involve known and unknown risks, uncertainties, and other factors, including those discussed under in our SEC filings, including under the heading “Item 1A. Risk Factors” in the Form 10-K for year ended December 31, 2018 we filed with the SEC and updated from time to time in our Form 10-Q filings and in our other public filings with the SEC. Any forward-looking statements contained in this release speak only as of its date. Soliton undertakes no obligation to update any forward-looking statements contained in this release to reflect events or circumstances occurring after its date or to reflect the occurrence of unanticipated events.
CONTACT: Joe Dorame, Joe Diaz & Robert Blum
Lytham Partners, LLC
602-889-9700
Source: Soliton, Inc.

Houston, TX – May 14, 2019 –Soliton, Inc., (NASDAQ: SOLY) (“Soliton” or the “Company”), a medical device company with a novel and proprietary platform technology licensed from The University of Texas on behalf of the MD Anderson Cancer Center (“MD Anderson”), today announced that the Japanese Patent Office has issued a notice of grant of patent Number 6503302, “Rapid Electrohydraulic Shockwave Generator”.
————————
Join our more than 200K fans here to follow the Company: https://soly-investors.com
————————
Clinical trials for tattoo removals have demonstrated that using the Company’s RAP device, in conjunction with a Q-Switched laser, allows for multiple passes of laser treatment in a single treatment session. The current standard of care for tattoo removal is to use a Q-Switched laser to ablate the tattoo ink particles into pieces small enough for the body’s natural processes to remove them. Independent studies have shown this standard treatment requires on average ten or more office visits to achieve acceptable results. In our own clinical trial using the RAP device in conjunction with a Q-Switched laser, patients experienced 75% to 100% removal of their tattoos in just three office visits.
Dr. Chris Capelli, President, CEO and Co-Founder of Soliton, said, “Our corporate patent portfolio continues to expand globally as a component of our overall intellectual property assets. This portfolio is essential to support the Company commercialization strategy which focuses both on domestic U.S. channels as well as aesthetic channels in a variety of international markets.” Dr. Capelli continued, “Additionally, clinical trials are under way to determine if our device may be effective at removing cellulite or accelerating current fat removal technologies.”
About Soliton, Inc.
Soliton, Inc. is a medical device company with a novel and proprietary platform technology licensed from MD Anderson. The Company’s first planned commercial product is designed to use rapid pulses of designed acoustic shockwaves in conjunction with existing lasers to reduce the number of treatment visits to remove unwanted tattoos (RAP device). In addition, higher energy versions of acoustic pulse devices are in early stages of development for potential stand-alone treatment of cellulite and other indications. Both products are investigational and are not available for sale in the United States.
For more information about the Company, please visit: http://www.soliton.com
Forward-Looking Statements
Some of the statements in this release are forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, Section 21E of the Securities Exchange Act of 1934 and the Private Securities Litigation Reform Act of 1995, which involve risks and uncertainties. Forward-looking statements in this press release include, without limitation, the ability of Soliton to secure adequate patent protection and whether future clinical trials will demonstrate the same results as past trials. These statements relate to future events, future expectations, plans and prospects. Although Soliton believes that the expectations reflected in such forward-looking statements are reasonable as of the date made, expectations may prove to have been materially different from the results expressed or implied by such forward-looking statements. Soliton has attempted to identify forward-looking statements by terminology including ”believes,” ”estimates,” ”anticipates,” ”expects,” ”plans,” ”projects,” ”intends,” ”potential,” ”may,” ”could,” ”might,” ”will,” ”should,” ”approximately” or other words that convey uncertainty of future events or outcomes to identify these forward-looking statements. These statements are only predictions and involve known and unknown risks, uncertainties, and other factors, including those discussed in our SEC filings, including under the heading “Item 1A. Risk Factors” in the Form 10-K for year ended December 31, 2018 we filed with the SEC and updated from time to time in our Form 10-Q filings and in our other public filings with the SEC. Any forward-looking statements contained in this release speak only as of its date. Soliton undertakes no obligation to update any forward-looking statements contained in this release to reflect events or circumstances occurring after its date or to reflect the occurrence of unanticipated events.
CONTACT: Joe Dorame, Joe Diaz & Robert Blum
Lytham Partners, LLC
602-889-9700
SOURCE: Soliton, Inc.

Replaceable Treatment Cartridge Paves the way for Future Recurring Revenue Stream
Houston, TX – May 1, 2019 –Soliton, Inc., (NASDAQ: SOLY) (“Soliton” or the “Company”), a medical device company with a novel and proprietary platform technology licensed from The University of Texas on behalf of the MD Anderson Cancer Center (“MD Anderson”), today announced that its manufacturing partner has delivered the first single-use cartridges capable of delivering the therapy targeting cellulite reduction.
The cartridge attaches to the treatment head and is designed to be used for a single patient cellulite treatment and then replaced. The cartridge is capable of delivering higher-powered acoustic pulses at greater depths than the Company’s tattoo removal cartridge, and both cartridges can be used with the same higher-powered pulse generating console, creating a true platform technology with a range of potential uses in the practicioner’s office. Neither the cartridge nor the device has been cleared by the FDA.
————————
Join our more than 200K fans here to follow the Company: https://soly-investors.com
————————
“We are very enthusiastic about the flexibility that our interchangeable treatment heads will bring to the practicioners that partner with us for the treatment of cellulite reduction and tattoo removal,” commented Dr. Chris Capelli, President, CEO and co-founder of Soliton. “ Our therapy will be delivered through single-use cartridges that are designed to be used for one patient treatment. Once our device is cleared by the FDA, we expect to deliver a recurring revenue stream and drive top-line growth for the Company.”
Dr. Capelli added: “We believe that this recurring revenue model will be readily adopted by the marketplace as other aesthetic technologies have led the way with pay-per-use models. We believe practicioners will be enthusiastic about our technology that, upon FDA clearance, could be used across multiple indications.”
About Soliton, Inc.
Soliton, Inc. is a medical device company with a novel and proprietary platform technology licensed from MD Anderson. The Company’s first planned commercial product is designed to use rapid pulses of designed acoustic shockwaves in conjunction with existing lasers to accelerate the removal of unwanted tattoos (RAP device). In addition, higher energy versions of acoustic pulse devices are in early stages of development for potential stand-alone treatment of cellulite and other indications. Both products are investigational and are not available for sale in the United States.
For more information about the Company, please visit: http://www.soliton.com
Forward-Looking Statements
Some of the statements in this release are forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, Section 21E of the Securities Exchange Act of 1934 and the Private Securities Litigation Reform Act of 1995, which involve risks and uncertainties. Forward-looking statements in this press release include, without limitation, the ability of the Soliton RAP technology, including the cellulite treatment cardridge, to prove safe and effective at reducing cellulite, for the Company’s devices to achieve FDA clearance for the tattoo and cellulite indications, and whether the Company’s intended revenue model will be accepted by the market. These statements relate to future events, future expectations, plans and prospects. Although Soliton believes that the expectations reflected in such forward-looking statements are reasonable as of the date made, expectations may prove to have been materially different from the results expressed or implied by such forward-looking statements. Soliton has attempted to identify forward-looking statements by terminology including ”believes,” ”estimates,” ”anticipates,” ”expects,” ”plans,” ”projects,” ”intends,” ”potential,” ”may,” ”could,” ”might,” ”will,” ”should,” ”approximately” or other words that convey uncertainty of future events or outcomes to identify these forward-looking statements. These statements are only predictions and involve known and unknown risks, uncertainties, and other factors, including those discussed under in our SEC filings, including under the heading “Item 1A. Risk Factors” in the Form 10-K for year ended December 31, 2018 we filed with the SEC and updated from time to time in our Form 10-Q filings and in our other public filings with the SEC. Any forward-looking statements contained in this release speak only as of its date. Soliton undertakes no obligation to update any forward-looking statements contained in this release to reflect events or circumstances occurring after its date or to reflect the occurrence of unanticipated events.
Source: Soliton, Inc.

2nd Gen Device Effectively Doubles the Peak Acoustic Pressure and Enables Deeper Penetration
Houston, TX – April 23, 2019 – Soliton, Inc., (NASDAQ: SOLY) (“Soliton” or the “Company”), a medical device company with a novel and proprietary platform technology licensed from The University of Texas on behalf of the MD Anderson Cancer Center (“MD Anderson”), today announced that its development team has completed final testing of the Company’s second generation rapid acoustic pulse (“Gen 2 RAP”) device at Sanmina, Inc., the Company’s global manufacturing partner.
The Gen 2 RAP device is intended to be used in Company’s upcoming pivotal registration clinical trial to submit to the FDA for 510(k) clearance of the device. This new device is designed to be capable of functioning both as the Gen 1 device does for the acceleration of tattoo removal, and as a stand-alone device for potential reduction of cellulite and other future indications. In this second model, the Gen 2 RAP should be capable of delivering higher-powered acoustic pulses at greater depths, making it a platform device with a wide range of potential future uses. This device has not been cleared by the FDA.
The treatment head of the Gen 2 RAP device is automated to allow continuous feeding of electrode material enabling the electrohydraulic pulse generator to operate for longer treatment times at higher and more consistent peak acoustic pressures. As well, the treatment head can now accommodate varying reflector designs to allow for treatment depths that are optimized for addressing the fibrotic structures that contribute to cellulite.
————————
Join our more than 200K fans here to follow the Company: https://soly-investors.com
————————
“Given the excitement surrounding the proof of concept cellulite trial results that are about to be presented to the scientific community,” commented Dr. Chris Capelli, President and CEO of Soliton, “we needed to be ready to launch an expanded clinical trial for the cellulite indication. The Gen 2 RAP device is the key to being able to start this expanded trial and seek FDA clearance.”
Dr. Capelli continued: “The Gen 2 RAP device represents the kind of multi-indication platform potential that we believe may make our RAP system an indispensable tool for clinicians.”
About Soliton, Inc.
Soliton, Inc. is a medical device company with a novel and proprietary platform technology licensed from MD Anderson. The Company’s first planned commercial product is designed to use rapid pulses of designed acoustic shockwaves in conjunction with existing lasers to accelerate the removal of unwanted tattoos (RAP device). In addition, higher energy versions of acoustic pulse devices are in early stages of development for potential stand-alone treatment of cellulite and other indications. Both products are investigational and are not available for sale in the United States.
For more information about the Company, please visit: http://www.soliton.com
Forward-Looking Statements
Some of the statements in this release are forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, Section 21E of the Securities Exchange Act of 1934 and the Private Securities Litigation Reform Act of 1995, which involve risks and uncertainties. Forward-looking statements in this press release include, without limitation, the ability of the Soliton RAP technology, including the Gen 2 RAP device, to prove safe and effective at reducing cellulite and to achieve FDA clearance for this indication. These statements relate to future events, future expectations, plans and prospects. Although Soliton believes that the expectations reflected in such forward-looking statements are reasonable as of the date made, expectations may prove to have been materially different from the results expressed or implied by such forward-looking statements. Soliton has attempted to identify forward-looking statements by terminology including ”believes,” ”estimates,” ”anticipates,” ”expects,” ”plans,” ”projects,” ”intends,” ”potential,” ”may,” ”could,” ”might,” ”will,” ”should,” ”approximately” or other words that convey uncertainty of future events or outcomes to identify these forward-looking statements. These statements are only predictions and involve known and unknown risks, uncertainties, and other factors, including those discussed under in our SEC filings, including under the heading “Item 1A. Risk Factors” in the Form 10-K for year ended December 31, 2018 we filed with the SEC and updated from time to time in our Form 10-Q filings and in our other public filings with the SEC. Any forward-looking statements contained in this release speak only as of its date. Soliton undertakes no obligation to update any forward-looking statements contained in this release to reflect events or circumstances occurring after its date or to reflect the occurrence of unanticipated events.
CONTACT:
Joe Dorame, Joe Diaz & Robert Blum
Lytham Partners, LLC
602-889-9700
SOURCE: Soliton, Inc.

Interview Will be Streamed Live at https://finance.yahoo.com during the 9:00AM EST Hour
Houston, TX – April 18, 2019 – Soliton, Inc., (NASDAQ: SOLY) (“Soliton” or the “Company”), a medical device company with a novel and proprietary platform technology licensed from The University of Texas on behalf of the MD Anderson Cancer Center (“MD Anderson”), today announced that its Executive Chairman, Walter Klemp, will give a live interview on Yahoo Finance on Monday, May 13, 2019.
As the Company has previously announced, data from its recently completed proof-of-concept clinical trial to study the safety and efficacy of a new version of its acoustic shockwave device for the treatment of moderate to severe cellulite will be presented to the scientific community at a national aesthetics conference, SCALE 2019 on May 11, 2019. This interview with Yahoo Finance will cover this new data and what it means for Soliton going forward.
————————
Join our more than 200K fans here to follow the Company: https://soly-investors.com
————————
“We’re pleased to announce that Yahoo Finance has selected us to be featured on their new live streaming network on ‘The First Trade’ on Monday, May 13, 2019 between 9AM – 10AM ET,” commented Walter Klemp, Executive Chairman of Soliton, Inc. “The focus will be to discuss our newly released cellulite clinical trial data. Yahoo Finance has a global audience of more than 90 million users each month and we’re excited to have the chance to show the world what the future may have in store for Soliton!”
About Soliton, Inc.
Soliton, Inc. is a medical device company with a novel and proprietary platform technology licensed from MD Anderson. The Company’s first planned commercial product is designed to use rapid pulses of designed acoustic shockwaves in conjunction with existing lasers to accelerate the removal of unwanted tattoos (RAP device). In addition, higher energy versions of acoustic pulse devices are in early stages of development for potential stand-alone treatment of cellulite and other indications. Both products are investigational and are not available for sale in the United States.
For more information about the Company, please visit: http://www.soliton.com
Forward-Looking Statements
Some of the statements in this release are forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, Section 21E of the Securities Exchange Act of 1934 and the Private Securities Litigation Reform Act of 1995, which involve risks and uncertainties. Forward-looking statements in this press release include, without limitation, viewership for ‘The First Trade’ on Yahoo Finance and the ability of Soliton’s acoustic shockwave device to reduce cellulite in the proof of concept clinical trial. These statements relate to future events, future expectations, plans and prospects. Although Soliton believes that the expectations reflected in such forward-looking statements are reasonable as of the date made, expectations may prove to have been materially different from the results expressed or implied by such forward-looking statements. Soliton has attempted to identify forward-looking statements by terminology including ”believes,” ”estimates,” ”anticipates,” ”expects,” ”plans,” ”projects,” ”intends,” ”potential,” ”may,” ”could,” ”might,” ”will,” ”should,” ”approximately” or other words that convey uncertainty of future events or outcomes to identify these forward-looking statements. These statements are only predictions and involve known and unknown risks, uncertainties, and other factors, including those discussed under in our SEC filings, including under the heading “Item 1A. Risk Factors” in the Form 10-K for year ended December 31, 2018 we filed with the SEC and updated from time to time in our Form 10-Q filings and in our other public filings with the SEC. Any forward-looking statements contained in this release speak only as of its date. Soliton undertakes no obligation to update any forward-looking statements contained in this release to reflect events or circumstances occurring after its date or to reflect the occurrence of unanticipated events.
CONTACT:
Joe Dorame,
Joe Diaz & Robert Blum
Lytham Partners, LLC
602-889-9700
SOURCE: Soliton, Inc.

Dr. Elizabeth Tanzi to Present Soliton’s Cellulite Data at Music City SCALE 2019 Conference in Nashville, TN
Houston, TX – April 15, 2019 –Soliton, Inc., (NASDAQ: SOLY) (“Soliton” or the “Company”), a medical device company with a novel and proprietary platform technology licensed from The University of Texas on behalf of the MD Anderson Cancer Center (“MD Anderson”), today announced that Dr. Elizabeth Tanzi of Capital Laser and Skin Care will present preliminary data from its completed cellulite clinical trial at the SCALE (Symposium for Cosmetic Advances and Laser Education) conference in Nashville, Tennessee to be held May 9-11, 2019.
The proof of concept study, conducted by Dr. Michael Kaminer at SkinCare Physicians in Boston, MA, in collaboration with Dr. Tanzi, was designed to evaluate the safety and efficacy of Soliton’s acoustic pulse device for the reduction of cellulite. The data presented will reflect results from a single acoustic pulse treatment, operating at a higher power level than the Company’s RAP device intended for tattoo removal, at the 12-week timepoint. Dr. Tanzi will also review preclinical data that appears to support what the Company believes may be a new method for treating cellulite.
————————
Join our more than 200K fans here to follow the Company: https://soly-investors.com
————————
“There has been a lot of anticipation surrounding the potential for our technology as a stand-alone treatment for cellulite,” commented Dr. Chris Capelli, President and CEO of Soliton, “especially considering the preclinical data suggesting that our unique acoustic pulses may be capable of achieving results only thought possible with invasive therapies. Considering that our technology is non-invasive and has been well tolerated by our trial participants, our goal has been to deliver clinically significant results without patient discomfort or downtime. We believe the upcoming presentation by Dr. Tanzi will be an important indicator of our progress and potential.”
Drs. Kaminer and Tanzi are members of Soliton’s Advisory Board.
About Soliton, Inc.
Soliton, Inc. is a medical device company with a novel and proprietary platform technology licensed from MD Anderson. The Company’s first planned commercial product is designed to use rapid pulses of designed acoustic shockwaves in conjunction with existing lasers to accelerate the removal of unwanted tattoos (RAP device). In addition, higher energy versions of acoustic pulse devices are in early stages of development for potential stand-alone treatment of cellulite and other indications. Both products are investigational and are not available for sale in the United States.
For more information about the Company, please visit: http://www.soliton.com
Forward-Looking Statements
Some of the statements in this release are forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, Section 21E of the Securities Exchange Act of 1934 and the Private Securities Litigation Reform Act of 1995, which involve risks and uncertainties. Forward-looking statements in this press release include, without limitation, the ability of Soliton’s acoustic shockwave device to reduce cellulite in the proof of concept clinical trial. These statements relate to future events, future expectations, plans and prospects. Although Soliton believes that the expectations reflected in such forward-looking statements are reasonable as of the date made, expectations may prove to have been materially different from the results expressed or implied by such forward-looking statements. Soliton has attempted to identify forward-looking statements by terminology including ”believes,” ”estimates,” ”anticipates,” ”expects,” ”plans,” ”projects,” ”intends,” ”potential,” ”may,” ”could,” ”might,” ”will,” ”should,” ”approximately” or other words that convey uncertainty of future events or outcomes to identify these forward-looking statements. These statements are only predictions and involve known and unknown risks, uncertainties, and other factors, including those discussed under in our SEC filings, including under the heading “Item 1A. Risk Factors” in the Form 10-K for year ended December 31, 2018 we filed with the SEC and updated from time to time in our Form 10-Q filings and in our other public filings with the SEC. Any forward-looking statements contained in this release speak only as of its date. Soliton undertakes no obligation to update any forward-looking statements contained in this release to reflect events or circumstances occurring after its date or to reflect the occurrence of unanticipated events.
CONTACT:
Joe Dorame, Joe Diaz & Robert Blum
Lytham Partners, LLC
602-889-9700
soly@lythampartners.com
Source: Soliton, Inc.

Houston, TX – April 9, 2019, — Soliton, Inc., (NASDAQ: SOLY) (“Soliton” or the “Company”), a medical device company with a novel and proprietary platform technology licensed from The University of Texas on behalf of the MD Anderson Cancer Center (“MD Anderson”), today announced it has completed the first evaluation timepoint for its proof of concept clinical trial for the treatment of cellulite. Results for a single acoustic shockwave treatment are being assessed by a blinded group of clinicians.
The study, conducted by Dr. Michael Kaminer at SkinCare Physicians in Boston, MA, in collaboration with Dr. Elizabeth Tanzi of Capital Laser and Skin Care, concluded evaluation of safety and efficacy for all patients through the initial 12-week time point. The study is designed to evaluate results at both the 12-week and 26-week timepoints from initial treatment in order to assess both near-term and long-term effects.
————————
Join our more than 200K fans here to follow the Company: https://soly-investors.com
————————
“We are pleased with how quickly this trial has progressed,” commented Dr. Chris Capelli, Soliton’s President and CEO. “We look forward to disclosing the independent assessment of the data from this trial in the near future. We believe the opportunity in the treatment of cellulite could be very significant for Soliton.”
Dr. Michael Kaminer of SkinCare Physicians added: “Having led the clinical development of Cellfina, the only treatment with FDA clearance for producing long-term reduction of cellulite, I am particularly aware of the unmet need in the treatment of cellulite. Having a non-invasive alternative treatment could provide a real benefit for patients while also expanding the breadth of services offered by clinicians.”
Drs. Kaminer and Tanzi are members of Soliton’s Scientific Advisory Board.
About Soliton, Inc.
Soliton, Inc. is a medical device company with a novel and proprietary platform technology licensed from MD Anderson. The Company’s first planned commercial product is designed to use rapid pulses of designed acoustic shockwaves in conjunction with existing lasers to accelerate the removal of unwanted tattoos (RAP device). In addition, higher energy versions of acoustic pulse devices are in early stages of development for potential stand-alone treatment of cellulite and other indications. Both products are investigational and are not available for sale in the United States.
For more information about the Company, please visit: http://www.soliton.com
Forward-Looking Statements
Some of the statements in this release are forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, Section 21E of the Securities Exchange Act of 1934 and the Private Securities Litigation Reform Act of 1995, which involve risks and uncertainties. Forward-looking statements in this press release include, without limitation, the ability of Soliton’s acoustic shockwave device to reduce cellulite in the proof of concept clinical trial. These statements relate to future events, future expectations, plans and prospects. Although Soliton believes that the expectations reflected in such forward-looking statements are reasonable as of the date made, expectations may prove to have been materially different from the results expressed or implied by such forward-looking statements. Soliton has attempted to identify forward-looking statements by terminology including ”believes,” ”estimates,” ”anticipates,” ”expects,” ”plans,” ”projects,” ”intends,” ”potential,” ”may,” ”could,” ”might,” ”will,” ”should,” ”approximately” or other words that convey uncertainty of future events or outcomes to identify these forward-looking statements. These statements are only predictions and involve known and unknown risks, uncertainties, and other factors, including those discussed under in our SEC filings, including under the heading “Item 1A. Risk Factors” in the Form 10-K for year ended December 31, 2018 we filed with the SEC and updated from time to time in our Form 10-Q filings and in our other public filings with the SEC. Any forward-looking statements contained in this release speak only as of its date. Soliton undertakes no obligation to update any forward-looking statements contained in this release to reflect events or circumstances occurring after its date or to reflect the occurrence of unanticipated events.
CONTACT:
Joe Dorame, Joe Diaz & Robert Blum
Lytham Partners, LLC
602-889-9700
Source: Soliton, Inc.

Houston, TX – April 1, 2019 – Soliton, Inc., (NASDAQ: SOLY) (“Soliton” or the “Company”), a medical device company with a novel and proprietary platform technology licensed from The University of Texas on behalf of the MD Anderson Cancer Center (“MD Anderson”), today summarized its efforts to conduct a proof of concept clinical trial for the treatment of cellulite based on Institutional Review Board (“IRB”) approval of the study. The study was initiated after positive results in animal studies indicated the potential for a higher-energy version of its acoustic shockwave technology to affect the factors contributing to the formation of cellulite.
The study treated each thigh of five patients (n=10) with a higher-energy version of Soliton’s acoustic shockwave device with the intent to evaluate safety and efficacy in the treatment of cellulite. The study is designed to evaluate results at both the 12-week and 26-week timepoints from initial treatment in order to assess both near-term and long-term effects.
—————————-
Join our more than 200K fans here to follow the Company: https://soly-investors.com
—————————-
“Our preclinical data led us to believe we could have a significant impact on the reduction of cellulite,” commented Dr. Chris Capelli, Soliton’s President and CEO. “This is especially significant since our technology should represent a non-invasive, pain free treatment that requires no anesthesia and involves no bruising, discomfort or downtime. If we are right, we believe this could be a major breakthrough in the treatment of cellulite.”
About Soliton, Inc.
Soliton, Inc. is a medical device company with a novel and proprietary platform technology licensed from MD Anderson. The Company’s first planned commercial product is designed to use rapid pulses of designed acoustic shockwaves in conjunction with existing lasers to accelerate the removal of unwanted tattoos (RAP device). In addition, higher energy versions of acoustic pulse devices are in early stages of development for potential stand-alone treatment of cellulite and other indications. Both products are investigational and are not available for sale in the United States.
For more information about the Company, please visit: http://www.soliton.com
Forward-Looking Statements
Some of the statements in this release are forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, Section 21E of the Securities Exchange Act of 1934 and the Private Securities Litigation Reform Act of 1995, which involve risks and uncertainties. Forward-looking statements in this press release include, without limitation, the ability of Soliton’s acoustic shockwave device to reduce cellulite in the proof of concept clinical trial. These statements relate to future events, future expectations, plans and prospects. Although Soliton believes that the expectations reflected in such forward-looking statements are reasonable as of the date made, expectations may prove to have been materially different from the results expressed or implied by such forward-looking statements. Soliton has attempted to identify forward-looking statements by terminology including ”believes,” ”estimates,” ”anticipates,” ”expects,” ”plans,” ”projects,” ”intends,” ”potential,” ”may,” ”could,” ”might,” ”will,” ”should,” ”approximately” or other words that convey uncertainty of future events or outcomes to identify these forward-looking statements. These statements are only predictions and involve known and unknown risks, uncertainties, and other factors, including those discussed under in our SEC filings, including under the heading “Item 1A. Risk Factors” in the Form 10-K for the year ended December 31, 2018 we filed with the SEC and updated from time to time in our other public filings with the SEC. Any forward-looking statements contained in this release speak only as of its date. Soliton undertakes no obligation to update any forward-looking statements contained in this release to reflect events or circumstances occurring after its date or to reflect the occurrence of unanticipated events.
CONTACT:
Joe Dorame, Joe Diaz & Robert Blum
Lytham Partners, LLC
602-889-9700
Source: Soliton, Inc.

510(k) Application Acceptance for Rapid Acoustic Pulse (“RAP”) device in tattoo removal
Houston, TX – March 27, 2019 – Soliton, Inc., (NASDAQ: SOLY) (“Soliton” or the “Company”), a medical device company with a novel and proprietary platform technology licensed from The University of Texas on behalf of the MD Anderson Cancer Center (“MD Anderson”), today announced that its 510(k) application for premarket clearance filed with the U.S. Food and Drug Administration (“FDA”) for its first generation Rapid Acoustic Pulse (“RAP”) tattoo removal device has cleared the agency’s acceptance review. The application now moves to a substantive review. The device is indicated as an accessory to the 1064 nm Q-Switched (pulsed) laser for black ink tattoo removal on the arms, legs and torso in Fitzpatrick Skin Type I-III individuals. Clinical trials have demonstrated that using the Company’s RAP device, in conjunction with a Q-Switched laser, allows for multiple passes of laser treatment in a single treatment session. The current standard of care for tattoo removal is to use a Q-Switched laser to ablate the tattoo ink particles into pieces small enough for the body’s natural processes to remove them, that independent studies have shown require on average ten or more office visits to achieve acceptable results. In our own clinical trial using the RAP device in conjunction with a Q-Switched laser, patients experienced 75% to 100% removal of their tattoos in just three office visits.
————————
Join our more than 200K fans here to follow the Company: https://soly-investors.com
————————
Dr. Christopher Capelli, president and CEO of Soliton, said, “We are very pleased with the progress of our premarket notification through the FDA review process.” Dr. Capelli continued, “Having found that our submission contained all the necessary elements and information, we look forward to the substantive review and FDA clearance of the RAP device.”
“We are excited about the prospects for our RAP technology as we enter the tattoo removal segment—estimated to be approximately $4.8 billion annually by 2023. And, we expect our higher energy version of this new technology will eventually take us into additional, and potentially bigger aesthetics markets. Because our technology relies upon replaceable cartridges for each treatment, we believe our business model will benefit from recurring revenue, allowing Soliton to share in the volume growth in the aesthetic category expected in the coming years,” said Dr. Capelli.
About Soliton, Inc.
Soliton, Inc. is a medical device company with a novel and proprietary platform technology licensed from MD Anderson. The Company’s first planned commercial product is designed to use rapid pulses of designed acoustic shockwaves in conjunction with existing lasers to accelerate the removal of unwanted tattoos (RAP device). In addition, higher energy versions of acoustic pulse devices are in early stages of development for potential stand-alone treatment of cellulite and other indications. Both products are investigational and are not available for sale in the United States.
For more information about the Company, please visit: http://www.soliton.com
Forward-Looking Statements
Some of the statements in this release are forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, Section 21E of the Securities Exchange Act of 1934 and the Private Securities Litigation Reform Act of 1995, which involve risks and uncertainties. Forward-looking statements in this press release include, without limitation, the ability of Soliton RAP to accelerate tattoo fading or fat removal and/or to reduce cellulite, whether future clinical trials related to the acceleration of existing fat removal technologies and cellulite are successful, the ability of Soliton’s RAP to receive FDA clearance, and the ability of Soliton’s higher energy device to be proven safe and effective in other aesthetic procedures. These statements relate to future events, future expectations, plans and prospects. Although Soliton believes that the expectations reflected in such forward-looking statements are reasonable as of the date made, expectations may prove to have been materially different from the results expressed or implied by such forward-looking statements. Soliton has attempted to identify forward-looking statements by terminology including ”believes,” ”estimates,” ”anticipates,” ”expects,” ”plans,” ”projects,” ”intends,” ”potential,” ”may,” ”could,” ”might,” ”will,” ”should,” ”approximately” or other words that convey uncertainty of future events or outcomes to identify these forward-looking statements. These statements are only predictions and involve known and unknown risks, uncertainties, and other factors, including those discussed under in our SEC filings, including under the heading “Risk Factors” in the Form 1-A we filed with the SEC on February 13, 2019. Any forward-looking statements contained in this release speak only as of its date. Soliton undertakes no obligation to update any forward-looking statements contained in this release to reflect events or circumstances occurring after its date or to reflect the occurrence of unanticipated events.
CONTACT:
Joe Dorame, Joe Diaz & Robert Blum
Lytham Partners, LLC
602-889-9700
Source: Soliton, Inc.

510(k) Application for Premarket Clearance filed with the FDA for Rapid Acoustic Pulse (“RAP”) device in tattoo removal
Houston, TX – March 21, 2019 – Soliton, Inc., (NASDAQ: SOLY) (“Soliton” or the “Company”),a medical device company with a novel and proprietary platform technology licensed from The University of Texas on behalf of the MD Anderson Cancer Center (“MD Anderson”), today announced that it has filed for 510(k) premarket clearance with the U.S. Food and Drug Administration (“FDA”) for its first generation Rapid Acoustic Pulse (“RAP”) tattoo removal device. The device is indicated as an accessory to the 1064 nm Q-Switched laser for black ink tattoo removal on the arms, legs and torso in Fitzpatrick Skin Type I-III individuals. Clinical trials have demonstrated that using the Company’s RAP device, in conjunction with a Q-switched laser, allows for multiple passes of laser treatment in a single treatment session, resulting in accelerated fading in comparison to stand-alone laser treatment. The current standard of care for tattoo removal is to use a Q-switched (pulsed) laser to ablate the tattoo ink particles into pieces small enough for the body’s natural processes to remove them. Unfortunately, this current method is highly inefficient, requiring up to 10 or more office visits to achieve acceptable results. A clinical trial has demonstrated that using our Rapid Acoustic Pulse (“RAP”) device in conjunction with a Q-switched laser has the potential to produce similar results in just 2 to 3 office visits.
————————
Join our more than 208K fans here to follow the Company: https://soly-investors.com
————————
Dr. Christopher Capelli, president and CEO of Soliton, said, “The submission of our 510(k) application for premarket clearance of our RAP device represents an important step in the commercialization of our products. We will be operating in a large industry segment that seeks the solutions our technology offers in terms of speed of tattoo removal and the potential for higher operating margins for the professional practices that adopt the RAP technology.”
“Our strategy is a razor/razorblade model with consumable products for single-use applications, generating recurring revenue that should allow Soliton to share in the volume growth that is expected in the coming years,” continued Dr. Capelli. “We believe this business model will serve the Company and our shareholders well as we enter into the tattoo removal segment – estimated to be approximately $4.8 billion annually by 2023 – and eventually into additional aesthetics markets. We are excited about the prospects for expanding the use of our RAP technology to address new markets and other aesthetic issues.”
About Soliton, Inc.
Soliton, Inc. is a medical device company with a novel and proprietary platform technology licensed from MD Anderson. The Company’s first planned commercial product is designed to use rapid pulses of designed acoustic shockwaves in conjunction with existing lasers to accelerate the removal of unwanted tattoos (RAP device). In addition, higher energy versions of acoustic pulse devices are in early stages of development for potential stand-alone treatment of cellulite and other indications. Both products are investigational and are not available for sale in the United States.
For more information about the Company, please visit: http://www.soliton.com
Forward-Looking Statements
Some of the statements in this release are forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, Section 21E of the Securities Exchange Act of 1934 and the Private Securities Litigation Reform Act of 1995, which involve risks and uncertainties. Forward-looking statements in this press release include, without limitation, the ability of Soliton RAP to accelerate tattoo fading or fat removal and/or to reduce cellulite, and whether future clinical trials related to the acceleration of existing fat removal technologies and cellulite are successful. These statements relate to future events, future expectations, plans and prospects. Although Soliton believes that the expectations reflected in such forward-looking statements are reasonable as of the date made, expectations may prove to have been materially different from the results expressed or implied by such forward-looking statements. Soliton has attempted to identify forward-looking statements by terminology including ”believes,” ”estimates,” ”anticipates,” ”expects,” ”plans,” ”projects,” ”intends,” ”potential,” ”may,” ”could,” ”might,” ”will,” ”should,” ”approximately” or other words that convey uncertainty of future events or outcomes to identify these forward-looking statements. These statements are only predictions and involve known and unknown risks, uncertainties, and other factors, including those discussed under in our SEC filings, including under the heading “Risk Factors” in the Form 1-A we filed with the SEC on February 13, 2019. Any forward-looking statements contained in this release speak only as of its date. Soliton undertakes no obligation to update any forward-looking statements contained in this release to reflect events or circumstances occurring after its date or to reflect the occurrence of unanticipated events.
Source: Soliton, Inc.

Houston, TX – March 19, 2019 – Soliton, Inc., (Nasdaq: SOLY) (“Soliton” or the “Company”), a medical device company with a novel and proprietary platform technology licensed from The University of Texas on behalf of the MD Anderson Cancer Center (“MD Anderson”), completed the human trials of its proprietary Rapid Acoustic Pulse (RAP) Device to support the Company’s FDA 510(k) submission. The Company conducted three rounds of human trials to study the use of the RAP device to accelerate tattoo fading.
————————
Join our more than 208K fans here to follow the Company: https://soly-investors.com
————————
“The human trials provided evidence of the potential for the RAP technology to accelerate tattoo fading,” said Dr. Chris Capelli, Soliton’s President and CEO. “The combination treatment of the RAP device and a laser outperformed a laser alone, showing an average of 80% fading after only two visits vs 44% fading for the laser alone. After 3 Soliton Multi-Pass treatments, 100% of the treated tattoos had a ‘Complete’ (76-100% faded) response; in comparison, only 17% of the tattoos treated with the Laser Only had a ‘Complete’ response.”
About Soliton, Inc.
Soliton, Inc. is a medical device company with a novel and proprietary platform technology licensed from MD Anderson. The Company’s first planned commercial product is designed to use rapid pulses of designed acoustic shockwaves in conjunction with existing lasers to accelerate the removal of unwanted tattoos (RAP device). In addition, higher energy versions of acoustic pulse devices are in early stages of development for potential stand-alone treatment of cellulite and other indications. Both products are investigational and are not available for sale in the United States.
For more information about the Company, please visit: http://www.soliton.com
Forward-Looking Statements
Some of the statements in this release are forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, Section 21E of the Securities Exchange Act of 1934 and the Private Securities Litigation Reform Act of 1995, which involve risks and uncertainties. Forward-looking statements in this press release include, without limitation, the ability of Soliton RAP to receive FDA clearance. These statements relate to future events, future expectations, plans and prospects. Although Soliton believes that the expectations reflected in such forward-looking statements are reasonable as of the date made, expectations may prove to have been materially different from the results expressed or implied by such forward-looking statements. Soliton has attempted to identify forward-looking statements by terminology including ”believes,” ”estimates,” ”anticipates,” ”expects,” ”plans,” ”projects,” ”intends,” ”potential,” ”may,” ”could,” ”might,” ”will,” ”should,” ”approximately” or other words that convey uncertainty of future events or outcomes to identify these forward-looking statements. These statements are only predictions and involve known and unknown risks, uncertainties, and other factors, including those discussed under in our SEC filings, including under the heading “Risk Factors” in the Form 1-A we filed with the SEC on February 13, 2019. Any forward-looking statements contained in this release speak only as of its date. Soliton undertakes no obligation to update any forward-looking statements contained in this release to reflect events or circumstances occurring after its date or to reflect the occurrence of unanticipated events.
CONTACT: Joe Dorame, Joe Diaz & Robert Blum
Lytham Partners, LLC
602-889-9700
SOURCE: Soliton, Inc.

Houston, TX – March 14, 2019 – Soliton, Inc., (Nasdaq: SOLY) (“Soliton” or the “Company”), a medical device company with a novel and proprietary platform technology licensed from The University of Texas on behalf of the MD Anderson Cancer Center (“MD Anderson”), highlights the Company’s important partnership with Sanmina Corporation (Nasdaq: SANM) to advance its acoustic shockwave technology into the commercialization phase in preparation for product launch.
————————
Join our more than 208K fans here to follow the Company: https://soly-investors.com
————————
Sanmina is a global contract electronics manufacturer and one of the world’s largest medical device manufacturers. Under the agreement, Sanmina will provide the design and testing to advance Soliton’s RAP device for use in future clinical trials and eventually to lay the foundation for a commercial launch of the Company’s products.
“Working with Sanmina in this commercialization phase not only provides us with world-class quality and documentation,” commented Dr. Chris Capelli, Soliton’s CEO, “but we also believe it will make for a smoother transition to manufacturing, which we expect to begin here in the US later this year.”
About Soliton, Inc.
Soliton, Inc. is a medical device company with a novel and proprietary platform technology licensed from MD Anderson. The Company’s first planned commercial product is designed to use rapid pulses of designed acoustic shockwaves in conjunction with existing lasers to accelerate the removal of unwanted tattoos (RAP device). In addition, higher energy versions of acoustic pulse devices are in early stages of development for potential stand-alone treatment of cellulite and other indications. Both products are investigational and are not available for sale in the United States.
For more information about the Company, please visit: http://www.soliton.com
Forward-Looking Statements
Some of the statements in this release are forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, Section 21E of the Securities Exchange Act of 1934 and the Private Securities Litigation Reform Act of 1995, which involve risks and uncertainties. Forward-looking statements in this press release include, without limitation, the ability of Soliton to receive clearance to market its devices and the ability of Sanmina Corporation to successfully commercialize Soliton’s technology. These statements relate to future events, future expectations, plans and prospects. Although Soliton believes that the expectations reflected in such forward-looking statements are reasonable as of the date made, expectations may prove to have been materially different from the results expressed or implied by such forward-looking statements. Soliton has attempted to identify forward-looking statements by terminology including ”believes,” ”estimates,” ”anticipates,” ”expects,” ”plans,” ”projects,” ”intends,” ”potential,” ”may,” ”could,” ”might,” ”will,” ”should,” ”approximately” or other words that convey uncertainty of future events or outcomes to identify these forward-looking statements. These statements are only predictions and involve known and unknown risks, uncertainties, and other factors, including those discussed under in our SEC filings, including under the heading “Risk Factors” in the Form 1-A we filed with the SEC on February 13, 2019. Any forward-looking statements contained in this release speak only as of its date. Soliton undertakes no obligation to update any forward-looking statements contained in this release to reflect events or circumstances occurring after its date or to reflect the occurrence of unanticipated events.
Source: Soliton, Inc.

Houston, TX – March 7, 2019 – Soliton, Inc., (NASDAQ: SOLY) (“Soliton” or the “Company”), a medical device company with a novel and proprietary platform technology licensed from The University of Texas on behalf of the MD Anderson Cancer Center (“MD Anderson”), discusses that their RAP device received institutional review board (“IRB”) approval as a non-significant risk device. Subsequent to receiving this status, the Company conducted several human clinical trials to study the use of the RAP device to accelerate tattoo fading and initiated a proof-of-concept trial in humans for the reduction of cellulite.
Soliton’s RAP device accelerates tattoo removal in part by providing dermal clearing of laser-generated vacuoles during laser treatment. This designation by the IRB allows the Company to use its device in human clinical trials.
The Institutional Review Board (IRB) is an FDA registered constituted group that has been formally designated to review and monitor biomedical research involving human subjects. In accordance with FDA regulations, an IRB has the authority to approve, require modifications (to secure approval), or disapprove research. This group review serves an important role in the protection of the rights and welfare of human research subjects.
“The NSR designation makes it very straight forward for us to conduct clinical trials with our technology,” commented Dr. Chris Capelli, Soliton’s President and CEO. “This applies not only to the clinical data we are presenting to the FDA with regard to accelerating tattoo removal, but potential future indications like cellulite reduction. We believe this allows us to move more quickly in developing Soliton RAP.”
About Soliton, Inc.
Soliton, Inc. is a medical device company with a novel and proprietary platform technology licensed from MD Anderson. The Company’s first planned commercial product will use rapid pulses of designed acoustic shockwaves to dramatically accelerate the removal of unwanted tattoos. The Company is based in Houston, Texas, and is actively engaged in bringing its Rapid Acoustic Pulse (“RAP”) device to the market. The Company believes this “Soliton” method can not only dramatically accelerate tattoo removal, but also has the potential to lower removal cost for patients, while increasing profitability to practitioners. Soliton has discovered other capabilities of the RAP technology during preclinical testing, including the potential to assist existing fat reduction technology in the reduction of fat as well as reducing the appearance of cellulite by creating mechanical stress at the cellular level and inducing significant collagen growth.
For more information about the Company, please visit: http://www.soliton.com
Forward-Looking Statements
Some of the statements in this release are forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, Section 21E of the Securities Exchange Act of 1934 and the Private Securities Litigation Reform Act of 1995, which involve risks and uncertainties. Forward-looking statements in this press release include, without limitation, the ability of Soliton RAP to receive FDA clearance, as well as reduce the appearance of cellulite in humans. These statements relate to future events, future expectations, plans and prospects. Although Soliton believes that the expectations reflected in such forward-looking statements are reasonable as of the date made, expectations may prove to have been materially different from the results expressed or implied by such forward-looking statements. Soliton has attempted to identify forward-looking statements by terminology including ”believes,” ”estimates,” ”anticipates,” ”expects,” ”plans,” ”projects,” ”intends,” ”potential,” ”may,” ”could,” ”might,” ”will,” ”should,” ”approximately” or other words that convey uncertainty of future events or outcomes to identify these forward-looking statements. These statements are only predictions and involve known and unknown risks, uncertainties, and other factors, including those discussed under in our SEC filings, including under the heading “Risk Factors” in the Form 1-A we filed with the SEC on February 13, 2019. Any forward-looking statements contained in this release speak only as of its date. Soliton undertakes no obligation to update any forward-looking statements contained in this release to reflect events or circumstances occurring after its date or to reflect the occurrence of unanticipated events.
Source: Soliton, Inc.

The Company qualifies for reduced or waived fees for medical device submissions with the FDA
Houston, TX – March 4, 2019 – Soliton, Inc., (NASDAQ: SOLY) (“Soliton” or the “Company”), a medical device company with a novel and proprietary platform technology licensed from The University of Texas M.D. on behalf of the MD Anderson Cancer Center (“MD Anderson”), today announced that it has received notice from the U.S. Food and Drug Administration (“FDA”) that the Company has qualified for the “Small Business” designation under the Medical Device User Fee Amendments (“MDUFA”). As a Small Business under the MDUFA, Soliton qualifies for a reduce or waived fee for medical device submissions made during the fiscal year 2019.
————————
Join our more than 208K fans here to follow the Company: https://soly-investors.com
————————
Dr. Christopher Capelli, president and CEO of Soliton, said, “We are pleased to have qualified for Small Business status allowing us to receive reduced or waived fees for our FDA submissions. We appreciate the opportunity to submit device applications for review and approval at more favorable rates. In certain submissions, the savings could be dramatic. This helps us to operate more efficiently. Our plan is to is to submit our Rapid Acoustic Pulse (“RAP”) device for tattoo removal for premarket clearance with the FDA under our new status in the near future.”
The MDUFA rates cover a number of submissions that are routinely made to the FDA for review and approval. The rate table assigns fees for the fiscal year – which ends on September 30, 2019 – for the application type submitted for review along with the Standard Fee and the Small Business fee for companies that qualify for the designation.
The table of 2019 MDUFA rates can be accessed at:
https://www.fda.gov/ForIndustry/UserFees/MedicalDeviceUserFee/ucm615142.htm
About Soliton, Inc.
Soliton, Inc. is a medical device company with a novel and proprietary platform technology licensed from MD Anderson. The Company’s first planned commercial product will use rapid pulses of designed acoustic shockwaves to dramatically accelerate the removal of unwanted tattoos. The Company is based in Houston, Texas, and is actively engaged in bringing this device to the market. The Company believes this “Soliton” method can not only dramatically accelerate tattoo removal, but also has the potential to lower removal cost for patients, while increasing profitability to practitioners, and to reduce the potential for unwanted side effects from current laser removal methods. Soliton has discovered other capabilities of the RAP technology during preclinical testing, including the potential to assist existing fat reduction technology in the reduction of fat as well as reducing the appearance of cellulite by creating mechanical stress at the cellular level and inducing significant collagen growth.
For more information about the Company, please visit: http://www.soliton.com
Forward-Looking Statements
Some of the statements in this release are forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, Section 21E of the Securities Exchange Act of 1934 and the Private Securities Litigation Reform Act of 1995, which involve risks and uncertainties. Forward-looking statements in this press release include, without limitation, the ability of Soliton RAP to accelerate tattoo fading or fat removal and/or to reduce cellulite, and whether future clinical trials related to the acceleration of existing fat removal technologies and cellulite are successful. These statements relate to future events, future expectations, plans and prospects. Although Soliton believes that the expectations reflected in such forward-looking statements are reasonable as of the date made, expectations may prove to have been materially different from the results expressed or implied by such forward-looking statements. Soliton has attempted to identify forward-looking statements by terminology including ”believes,” ”estimates,” ”anticipates,” ”expects,” ”plans,” ”projects,” ”intends,” ”potential,” ”may,” ”could,” ”might,” ”will,” ”should,” ”approximately” or other words that convey uncertainty of future events or outcomes to identify these forward-looking statements. These statements are only predictions and involve known and unknown risks, uncertainties, and other factors, including those discussed under in our SEC filings, including under the heading “Risk Factors” in the Form 1-A we filed with the SEC on February 13, 2019. Any forward-looking statements contained in this release speak only as of its date. Soliton undertakes no obligation to update any forward-looking statements contained in this release to reflect events or circumstances occurring after its date or to reflect the occurrence of unanticipated events.
Source: Soliton, Inc.

Rapid Acoustic Pulse device granted patent in China; bringing a total of 38 issued or pending patents
Houston, TX – February 26, 2019 – Soliton, Inc., (Nasdaq: SOLY), (“Soliton” or the “Company”), a medical technology company that has developed a new acoustic shockwave device, today announced that the National Intellectual Property Administration (Chinese Patent Office) has issued a notice of grant for Chinese Patent Application No. 201280041817.2, based on International Application No.PCT/IS2012/046674; Entitled “Apparatus for Generating Therapeutic Shockwaves And Applications of Same,” by Christopher C. Capelli.
Dr. Chris Capelli, CEO of Soliton, said, “The granting of this patent is an important milestone for Soliton. With the issuance of this patent in the People’s Republic of China (“PRC”) for our leading-edge Rapid Acoustic Pulse (“RAP”) technology and with our pending United States patents, we are well positioned to develop two of the major consumer markets in the world. We have a strong portfolio that now numbers 38 issued and pending patents that we believe provides Soliton a meaningful competitive advantage in removing unwanted tattoos in about one-third of the time of traditional procedures, with less pain and discomfort, and at a lower overall cost.”
Soliton has discovered other capabilities of the RAP technology during preclinical testing, including the potential to assist existing fat reduction technology in the reduction of fat as well as potentially reducing the appearance of cellulite by creating mechanical stress at the cellular level and inducing significant collagen growth. Importantly, this potential indication could position Soliton RAP as a stand-alone device, without the need for lasers or other procedures.
Dr. Capelli concluded, “If our ongoing clinical trials prove to be effective in cellulite and fat removal, we will dramatically expand our addressable markets. We are excited with the opportunities ahead in the coming years.”
About Soliton, Inc.
Soliton, Inc. is a medical device company with a novel and proprietary platform technology licensed from MD Anderson. The Company’s first planned commercial product will use rapid pulses of designed acoustic shockwaves to dramatically accelerate the removal of unwanted tattoos. The Company is based in Houston, Texas, and is actively engaged in bringing this device to the market. The Company expects to file for premarket clearance with the U.S. Food and Drug Administration (“FDA”) for its’ first device in the first quarter of 2019 and expects to receive clearance to market the device in mid 2019. This initial filing is limited to the Company’s device used in conjunction with the 1064 nm Q-switched laser to enable effective multiple pass laser treatments in a single office session to accelerate removal of black tattoos on the arms, legs and torso in Fitzpatrick Skin Type I-III individuals. While the Company believes its’ technology has many potential applications, the Company has initially focused on the removal of tattoos, where both animal and human studies have shown promising results. The current standard of care for tattoo removal is to use a Q-switched (pulsed) laser to ablate the tattoo ink particles into pieces small enough for the body’s natural processes to remove them. Unfortunately, this current method is highly inefficient, requiring up to 10 or more office visits to achieve acceptable results. A clinical trial has demonstrated that using the Company’s RAP device, in conjunction with a Q-switched laser, has the potential to produce similar results in just 2 to 3 office visits versus the industry average of 10 or more with a stand-alone laser. The Company believes this “Soliton” method can not only dramatically accelerate tattoo removal, but also has the potential to lower removal cost for patients, while increasing profitability to practitioners, and to reduce the potential for unwanted side effects from current laser removal methods.
For more information about the Company, please visit: http://www.soliton.com
Forward-Looking Statements
Some of the statements in this release are forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, Section 21E of the Securities Exchange Act of 1934 and the Private Securities Litigation Reform Act of 1995, which involve risks and uncertainties. Forward-looking statements in this press release include, without limitation, the ability of Soliton RAP to accelerate tattoo fading or fat removal and/or to reduce cellulite, and whether future clinical trials related to the acceleration of existing fat removal technologies and cellulite are successful. These statements relate to future events, future expectations, plans and prospects. Although Soliton believes that the expectations reflected in such forward-looking statements are reasonable as of the date made, expectations may prove to have been materially different from the results expressed or implied by such forward-looking statements. Soliton has attempted to identify forward-looking statements by terminology including ”believes,” ”estimates,” ”anticipates,” ”expects,” ”plans,” ”projects,” ”intends,” ”potential,” ”may,” ”could,” ”might,” ”will,” ”should,” ”approximately” or other words that convey uncertainty of future events or outcomes to identify these forward-looking statements. These statements are only predictions and involve known and unknown risks, uncertainties, and other factors, including those discussed under in our SEC filings, including under the heading “Risk Factors” in the Form 1-A we filed with the SEC on February 13, 2019. Any forward-looking statements contained in this release speak only as of its date. Soliton undertakes no obligation to update any forward-looking statements contained in this release to reflect events or circumstances occurring after its date or to reflect the occurrence of unanticipated events.
CONTACT:
Joe Dorame, Joe Diaz & Robert Blum
Lytham Partners, LLC
602-889-9700
Source: Soliton, Inc.

NEW YORK, HOUSTON and LOS ANGELES, Feb. 19, 2019 – Soliton, Inc. (NASDAQ: SOLY) (“Soliton” or the “Company”), a pre-revenue stage medical device company with a novel and proprietary platform technology licensed from The University of Texas on behalf of the MD Anderson Cancer Center (“MD Anderson”), today announced that its shares have commenced trading on the Nasdaq Capital Market under the symbol “SOLY”. Soliton closed its Regulation A+ Tier 2 offering (the “Offering”) by selling 2,172,591shares of its common stock at the IPO price of $5.00 each, resulting in aggregate gross proceeds of $10,862,955, before deducting underwriting commissions and other related expenses. Boustead Securities, LLC (“Boustead”) was the sole underwriter of the Soliton initial public offering (“IPO”), the California-based investment bank’s second Nasdaq IPO of 2019.
“We are thrilled to begin trading on Nasdaq,” said Soliton CEO Chris Capelli. “The closing of Soliton’s IPO marks the beginning of a new chapter for our company and its dedicated investors, both long-term and those that have come on board in this Nasdaq IPO. We will continue to push forward with our proprietary technology for tattoo removal and for cellulite reduction by completing further clinical and preclinical trials before bringing the Soliton’s Rapid Acoustic Pulse (“RAP”) shockwave device to a large addressable market,” Capelli stated.
“Congratulations to Soliton CEO Chris Capelli and the entire Soliton team for a promising trading debut on Nasdaq,” said Boustead’s Head of Equity Capital Markets, Dan McClory. “The successful completion of the first Reg A+ IPO on Nasdaq or the NYSE in over one year by any company is a particularly important milestone, and it says a lot about the resilience and commitment of Soliton, its investors, advisors, and underwriter,” McClory concluded.
The Company intends to use the net proceeds of the IPO offering primarily to develop and commercialize the RAP device; conduct further clinical trials for new indications; pay license fees and fund research and development; and for general working capital.
Soliton’s patented RAP device uses acoustic shockwaves that, in clinical trials, accelerated the speed of tattoo removal when used in conjunction with lasers, delivering results in as little as 2 to 3 treatments versus the 10 to 12 average with the current standard of care lasers alone. In addition to tattoo removal, Soliton discovered other capabilities of its technology during preclinical testing. Among them, the Company observed that their RAP device may have the potential to improve skin laxity as well as the appearance of cellulite by creating mechanical stress at the cellular level and inducing collagen growth. These clinical and preclinical trials are in early stages and intended as a proof-of-concept and there are no assurances that the trials will have a successful outcome.
An offering circular on Form 1-A relating to this U.S. offering was filed with the Securities and Exchange Commission (“SEC”) and was qualified by the SEC as of November 27, 2018. The offering of these securities was made only by means of an offering circular on Form 1-A. The final offering circular is available at https://www.flashfunders.com/soliton, https://www.sec.gov or may be obtained from Boustead Securities at +1 (949) 502-4409 or offerings@bousted1828.com
About the Company
Soliton, Inc. is a pre-revenue stage medical device company with a novel and proprietary platform technology licensed from MD Anderson. The Company’s first commercial product uses rapid pulses of designed acoustic shockwaves to dramatically accelerate the removal of unwanted tattoos. The Company is based in Houston, Texas, and is actively engaged in bringing this device to the market. The Company expects to file for premarket clearance with the U.S. Food and Drug Administration (“FDA”) for its first device in the first quarter of 2019 and expects to receive clearance to market the device in mid-2019. For more information about the Company, please visit: http://www.soliton.com.
About Boustead Securities, LLC
Boustead Securities, LLC (“Boustead”) is an investment banking firm that executes and advises on IPOs, mergers and acquisitions, capital raises and restructuring assignments in a wide array of industries, geographies and transactions, for a broad client base. Boustead’s core value proposition is the ability to create opportunity through innovative solutions and tenacious execution. With experienced professionals in the United States and around the world, Boustead’s team moves quickly and provides a broad spectrum of sophisticated financial advice and services. For more information about Boustead, please visit www.boustead1828.com
CONTACT:
Boustead Securities, LLC:
Dan McClory, Head of Equity Capital Markets
+1 (949) 502 4408
About FinTech Global Markets, Inc.
FinTech Global Markets, Inc. (“FTGM”) was founded in 2012 and is headquartered in Southern California. FTGM owns and operates a FINRA member broker-dealer, FinTech Clearing, LLC; FlashFunders Shareholder Services, LLC, a SEC-registered transfer agent; FlashFunders Funding Portal, LLC, a FINRA member funding portal; and two investment advisors, Maco.la Management, Inc. and Initiate Advisors, LLC. Through these subsidiaries, FTGM operates a U.S.-based online securities platform whose underlying technology and regulatory infrastructure are designed to enable issuers to engage in Regulation D, Regulation A (known as Reg A+), Regulation S, Regulation CF and fully registered S-1 and F-1 Initial Public Offerings on NASDAQ in compliance with applicable federal, state and non-U.S. securities laws. www.flashfunders.com
This press release is neither an offer to sell nor a solicitation of an offer to buy any securities of the Company, including without limitation the common stock nor shall such securities be offered or sold in the United States absent registration or an applicable exemption from registration, nor shall there be any offer, solicitation or sale of any of the Company’s securities in any state or jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of such state or jurisdiction.
Regulation A+ Offerings
An Offering Circular regarding Soliton, Inc. (“Soliton”) has been filed with the Securities and Exchange Commission (“SEC”). The SEC has qualified the Soliton Offering Circular, which only means that we may make sales of the securities described in its Offering Circular. It does not mean that the SEC has approved, passed upon the merits, or passed upon the accuracy or completeness of the information in Soliton’s Offering Circular. You may obtain copies of the Offering Circular for Soliton here: https://www.sec.gov/Archives/edgar/data/1548187/000162827918000315/soliton253g2.htm
Liquidity Risk-Regulation A+ Offerings
An investment in Soliton has a high degree of risk, including, but not limited to, a small equity market capitalization and lack of significant public float, which may impair the liquidity of these investments. Soliton can make no assurances about the success of its products, licensing or marketing efforts; consequently, investors in Soliton may lose some or all of their investments.
Safe Harbor Statement
The Company has made statements in this press release that are considered “forward-looking statements” which are usually identified by the use of words such as “anticipates,” “believes,” “estimates,” “expects,” “intends,” “may,” “plans,” “projects,” “seeks,” “should,” “will,” and variations of such words or similar expressions. All statements other than statements of historical fact in this press release are forward-looking statements. These forward-looking statements reflect our current views about our plans, intentions, expectations, strategies and prospects, which are based on the information currently available to us and on assumptions we have made. Although we believe that our plans, intentions, expectations, strategies and prospects as reflected in or suggested by those forward-looking statements are reasonable, we can give no assurance that the plans, intentions, expectations or strategies will be attained or achieved. Furthermore, actual results may differ materially from those described in the forward-looking statements and will be affected by a variety of risks and factors that are beyond our control. For further discussion of the factors that could affect outcomes, please refer to the risk factors set forth in the “Risk Factors” section of the Final Offering Circular. We assume no obligation to update publicly any forward-looking statements, whether as a result of new information, future events or circumstances, or changes in the Company’s expectations, except as may be required by law. Although the Company believes that the expectations expressed in these forward-looking statements are reasonable, it cannot assure you that such expectations will turn out to be correct, and the Company cautions investors that actual results may differ materially from the anticipated results.
CONTACT:
Joe Dorame, Joe Diaz & Robert Blum
Lytham Partners, LLC
602-889-9700
Source: Soliton, Inc.
Soliton, Inc. is a medical device company with a novel and proprietary platform technology licensed from MD Anderson. The Company’s first commercial product uses rapid pulses of designed acoustic shockwaves to dramatically accelerate the removal of unwanted tattoos. The Company is based in Houston, Texas, and is actively engaged in bringing this device to the market. The Company expects to file for premarket clearance with the U.S. Food and Drug Administration (“FDA”) for its’ first device in the first quarter of 2019 and expects to receive clearance to market the device in mid 2019. This initial filing is limited to the Company’s device used in conjunction with the 1064 nm Q-switched laser to enable effective multiple pass laser treatments in a single office session to accelerate removal of black tattoos on the arms, legs and torso in Fitzpatrick Skin Type I-III individuals. While the Company believes its’ technology has many potential applications, the Company has initially focused on the removal of tattoos, where both animal and human studies have shown promising results. The current standard of care for tattoo removal is to use a Q-switched (pulsed) laser to ablate the tattoo ink particles into pieces small enough for the body’s natural processes to remove them. Unfortunately, this current method is highly inefficient, requiring up to 10 or more office visits to achieve acceptable results. A clinical trial has demonstrated that using the Company’s RAP device, in conjunction with a Q-switched laser, has the potential to produce similar results in just 2 to 3 office visits. The Company believes this “Soliton” method can not only dramatically accelerate tattoo removal, but also has the potential to lower removal cost for patients, while increasing profitability to practitioners, and to reduce the potential for unwanted side effects from current laser removal methods.
Soliton is developing breakthrough acoustic shockwave technology to address multiple multibillion-dollar market opportunities.
Our clinically proven Rapid Acoustic Pulse (RAP) device uses proprietary acoustic shockwaves to dramatically accelerate the removal of tattoos. We’ve also recently discovered that our device may have the potential to reduce cellulite and blast apart fat cells.
We are science-driven and led by executives and medical advisors from some of the world’s most successful aesthetic and medical device companies. Let us show you our plans to revolutionize the aesthetics industry.
We’ve spent over 25 million dollars over the past 5 years developing a breakthrough technology that dramatically accelerates the removal of tattoos. Our devices use very high energy, in the range of 3,000 volts at nearly 3,000 amps, to form acoustic shockwaves at 100 times per second. Our core team is comprised of life science veterans, with more than 150 years of life science experience, multiple device clearances, and multiple national and international aesthetic device launches.
Source: https://www.soliton.com/

